Figures & data
Figure 1 Study design.
Abbreviations: IND/GLY, indacaterol/glycopyrronium; o.d., once daily; V, visit.
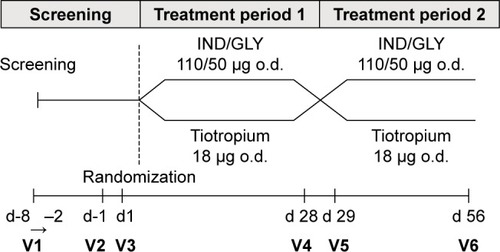
Figure 2 Patient disposition (full analysis set).
Abbreviation: IND/GLY, indacaterol/glycopyrronium.
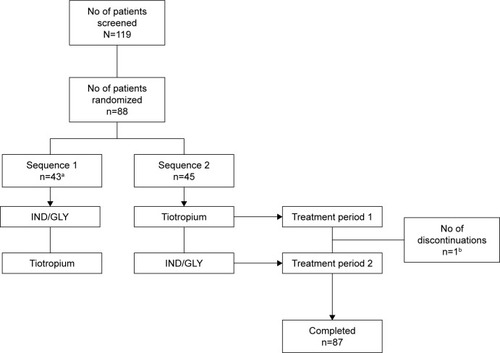
Table 1 Demographic and baseline characteristics (full analysis set)
Figure 3 FEV1 (L) 1 h post-inhalation after 4 weeks of each treatment (full analysis set).
Abbreviations: FEV1, forced expiratory volume in 1 s; IND/GLY, indacaterol/glycopyrronium.
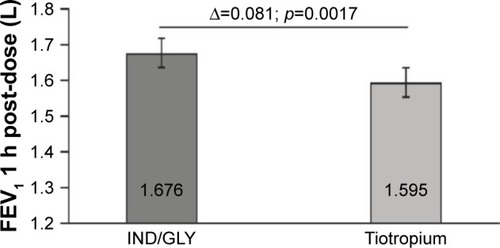
Table 2 Treatment preference after receiving both the treatments (full analysis set)
Figure 4 Very important/important subjective reasons cited by patients and physicians for a preference for IND/GLY or tiotropium (full analysis set).
Abbreviation: IND/GLY, indacaterol/glycopyrronium.
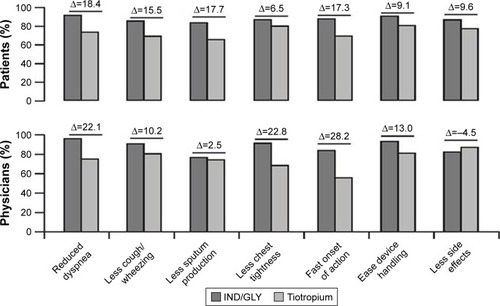
Figure 5 Patients very satisfied/satisfied with their treatment (full analysis set).
Abbreviation: IND/GLY, indacaterol/glycopyrronium.
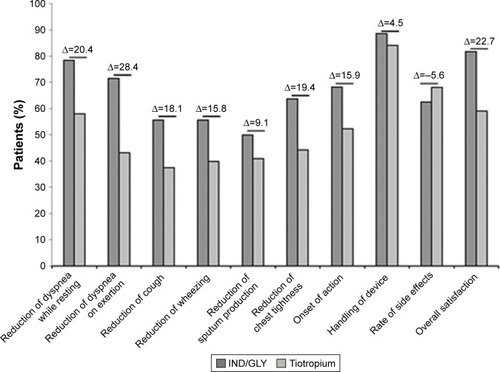
Figure 6 TSQM-9 questionnaire results by domain (full analysis set).
Abbreviations: IND/GLY, indacaterol/glycopyrronium; TSQM-9, Treatment Satisfaction Questionnaire for Medication.
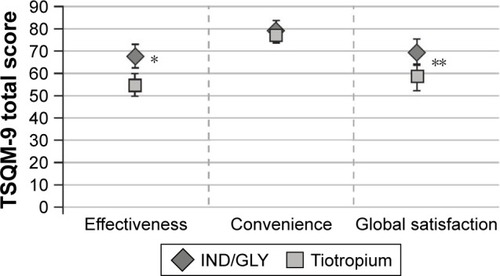
Figure 7 Improvement in FEV1 (L) 1 h post-inhalation based on medication preference (full analysis set).
Abbreviations: FEV1, forced expiratory volume in 1 s; IND/GLY, indacaterol/glycopyrronium.
Table 3 Incidences of TEAEs reported by ≥2.5% of patients in either group (safety analysis set)
