Figures & data
Figure 1 (A) isPLA of phosphorylated p38α in alveolar macrophages of lung tissue from COPD patients and controls. Data are representative of two experiments. (B) AZD7624 inhibition of LPS-stimulated pro-inflammatory cytokine TNF-α in human alveolar macrophages. Data are presented as mean ± SEM of n=4. AZD7624 attenuation of (C) sputum neutrophil % differential and (D) TNF-α changes from baseline compared with placebo in the human LPS challenge study. Data are presented as mean ± SEM of 24–27 subjects per treatment group. ap<0.001 vs placebo.
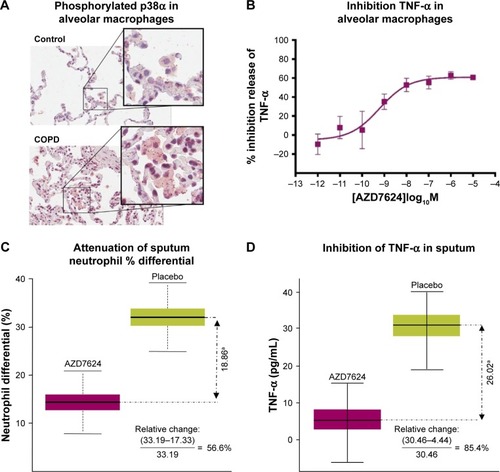
Table 1 Adverse events, Proof of Mechanism studyTable Footnotea
Table 2 Baseline demographics, COPD Proof of Principle study
Figure 2 Patient disposition. aInformed consent received. bOne patient was excluded from the analysis following review of protocol deviations owing to a lack of source data and Good Clinical Practice compliance. cPatient withdrawal recorded as “other” by study site investigators; one patient was unable to complete the study visits owing to terminal illness of family member; another patient was withdrawn by the study site investigator after a reassessment of patient eligibility (on day 4 after randomization). dCompletion status of two patients was not recorded.
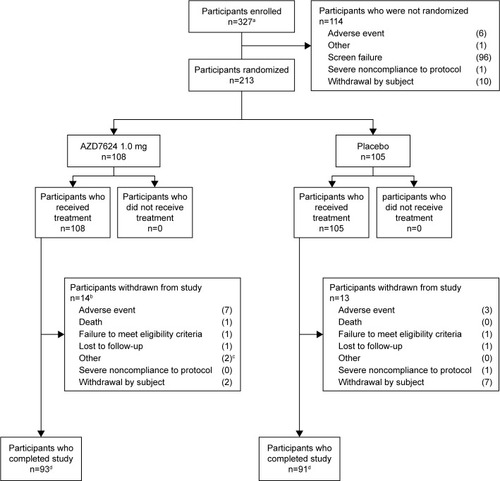
Table 3 Key secondary endpoints, COPD Proof of Principle study
Figure 3 (A) Time to first event of ExDo (HR =1.39, 95% CI 0.81, 2.40; p=0.24). (B) Time to first moderate or severe COPD exacerbation (HR =1.53, 95% CI 0.85, 2.76; p=0.15). (C) Time to first event of ExDo ≤2% eosinophils (HR =0.93, 95% CI 0.42, 2.05; p=0.85). (D) Time to first event of ExDo >2% eosinophils (HR =2.20, 95% CI 0.95, 5.09; p=0.07).
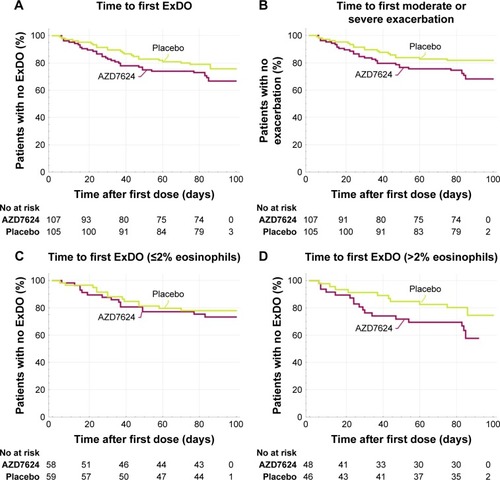
Table 4 AEs and serious AEs, COPD Proof of Principle studyTable Footnotea
