Figures & data
Table 1 Baseline demographics and clinical characteristics (efficacy analysis set)
Figure 1 Patient disposition.
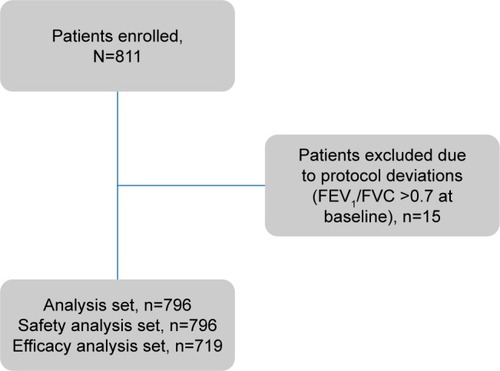
Figure 2 COPD severity as per GOLD classification based on (A) airflow limitation and (B) GOLD groups (efficacy analysis set).
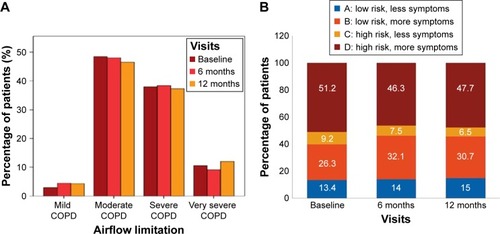
Figure 3 Distribution of patients according to (A) CAT scores and (B) mMRC scores (efficacy analysis set).
Abbreviations: CAT, COPD assessment test; COPD, chronic obstructive pulmonary disease; mMRC, modified Medical Research Council.
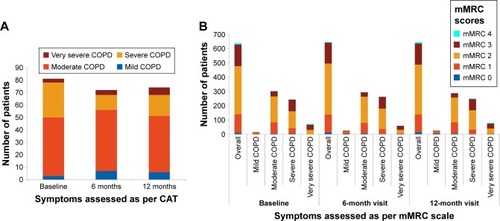
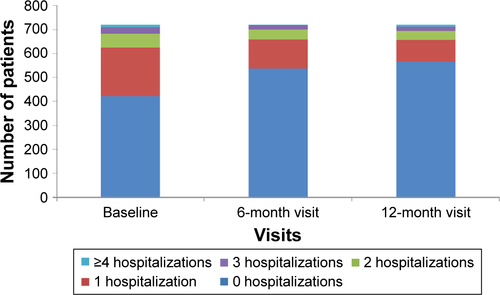
Figure 4 Proportion of patients experiencing exacerbations (efficacy analysis set).
Abbreviation: COPD, chronic obstructive pulmonary disease.
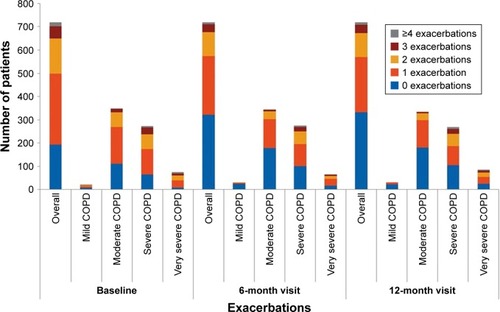
Figure S1 Treatment patterns over 12 months (efficacy analysis set).
Note: Data are presented as percentage of patients.
Abbreviations: FDC, fixed-dose combination; ICS, inhaled corticosteroid; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; PDE-4, phosphodiesterase-4; SABA, short-acting β2-agonists; SAMA, short-acting muscarinic antagonists.
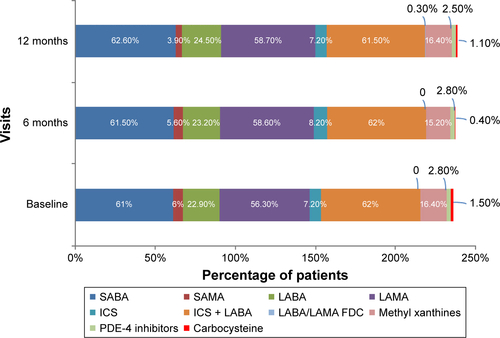
Figure S2 Long-acting inhaled therapy and agreement of treatment with GOLD guidelines (A and B) at baseline; (C and D) at 6 months; (E and F) at 12 months (efficacy analysis set).
Notes: Data are presented as number of patients (A, C, and E) and percentage of patients (B, D, and F). A: GOLD group A; B: GOLD group B; C: GOLD group C; D: GOLD group D.
Abbreviations: GOLD, Global Initiative for Chronic Obstructive Lung Disease; ICS, inhaled corticosteroid; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist.
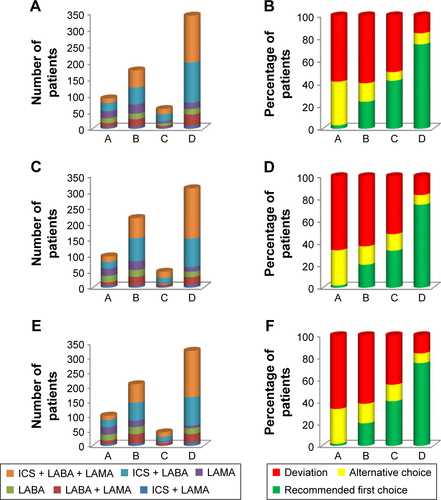
Table S1 List of study centers and contributors
Table S2 Adverse events and serious adverse events, according to system organ class