Figures & data
Figure 1 Patient disposition.
Abbreviations: EOD, every other day; LTFU, lost to follow-up; OD, once daily; ROF, roflumilast; 250, 250 µg; 500, 500 µg.
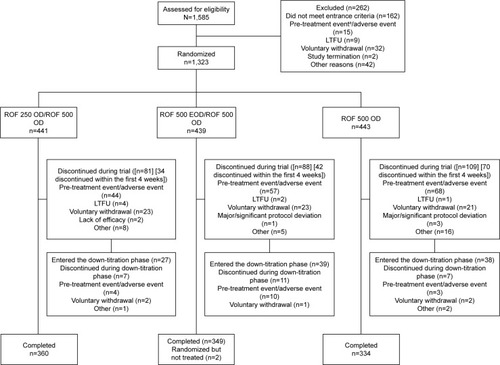
Table 1 Baseline patient characteristics
Figure 2 (A) Probability of patients continuing the 12-week trial for any reason (SAS). (B) Percentage of patients discontinuing for any reason.
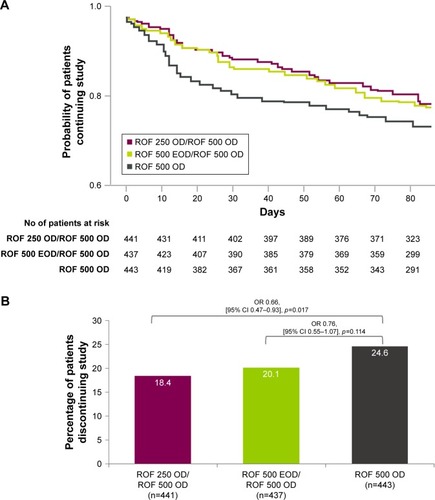
Figure 3 Summary of AEs of interest.
Abbreviations: AE, adverse event; CI, confidence interval; EOD, every other day; OD, once daily; OR, odds ratio; ROF, roflumilast; 250, 250 µg; 500, 500 µg.
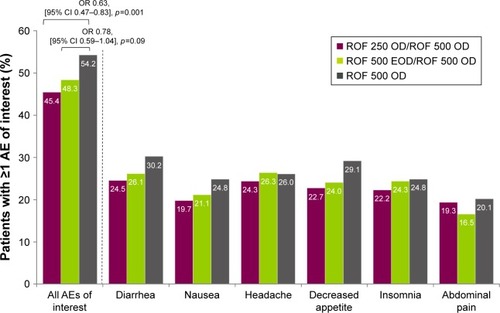
Figure 4 Mean change in body weight.
Abbreviations: EOD, every other day; OD, once daily; ROF, roflumilast; SD, standard deviation; 250, 250 µg; 500, 500 µg.
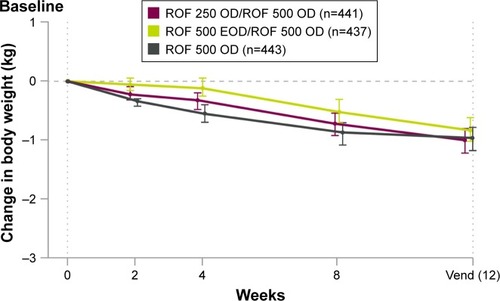
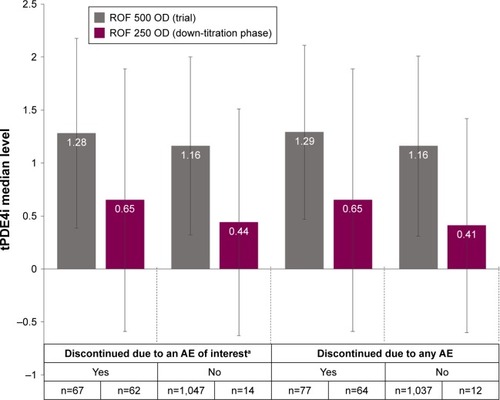
Figure 5 Comparison of tPDE4i levels achieved with ROF 500 µg or 250 µg according to whether patients were able to tolerate the dose.
Abbreviations: AE, adverse event; CI, confidence interval; OD, once daily; popPK, population pharmacokinetics; ROF, roflumilast; tPDE4i, total phosphodiesterase-4 inhibitory activity; 250, 250 µg; 500, 500 µg.