Figures & data
Table 1 Baseline demographics and clinical characteristics (PK sub-study)
Table 2 PK parameters of glycopyrronium and formoterol on Day 1 and Week 12 (PK population)
Figure 1 Plasma concentrations after single and chronic dosing.
Abbreviations: GFF, glycopyrronium/formoterol fumarate dihydrate; MDI, metered dose inhaler; GP, glycopyrronium; FF, formoterol fumarate dihydrate; PK, pharmacokinetic.
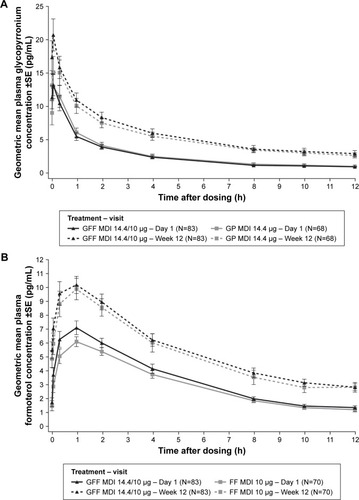
Table 3 Accumulation ratios (Week 12/Day 1) for glycopyrronium and formoterol following GFF MDI and GP MDI or FF MDI administration (PK population)
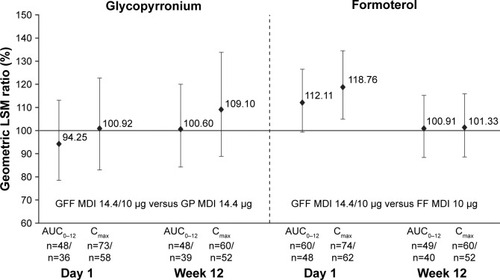
Figure 2 Relative bioavailability for glycopyrronium and formoterol following GFF MDI and GP MDI or FF MDI administration (GFF MDI/monocomponent MDI) (PK population).
Abbreviations: GFF, glycopyrronium/formoterol fumarate dihydrate; MDI, metered dose inhaler; GP, glycopyrronium; FF, formoterol fumarate dihydrate; PK, pharmacokinetic; LSM, least squares mean.