Figures & data
Table 1 Renal impairment categories
Table 2 Demographic and baseline patient characteristics by renal impairment category
Table 3 Summary of adverse events by baseline renal impairment
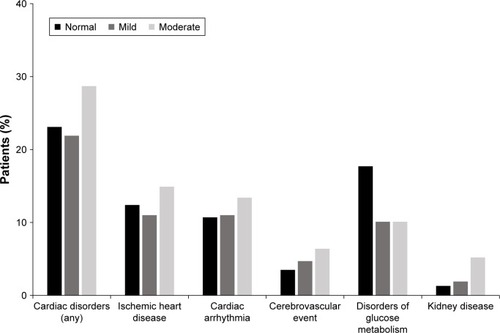
Figure 2 Incidence of adverse events and serious adverse events by renal impairment category.
Abbreviations: AE, adverse event; Olo, olodaterol; SAE, serious AE; Tio, tiotropium.
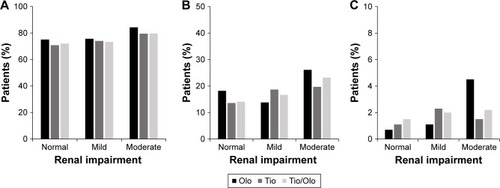
Figure 3 Discontinuation of study medication by renal impairment category.
Abbreviations: Olo, olodaterol; Tio, tiotropium.
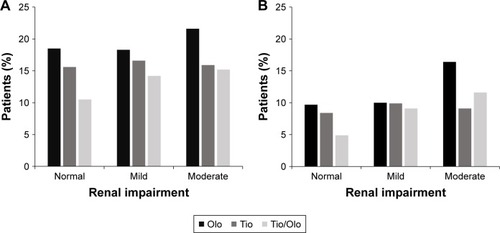
Table 4 Adverse events in individual patients with severe renal impairment at baseline
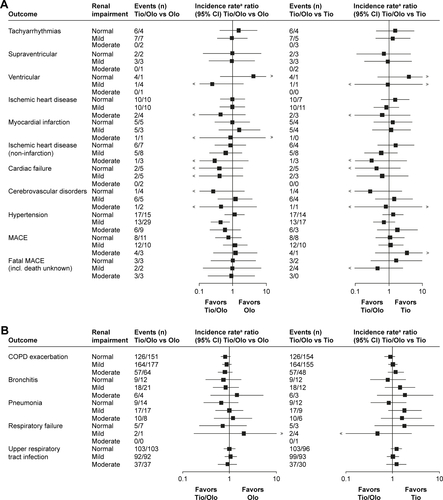
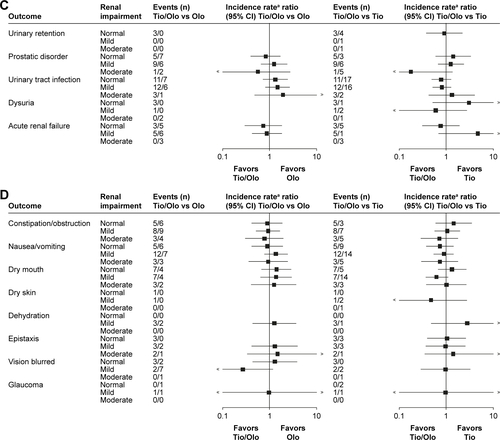
Figure 4 Exposure-adjusted incidence rate ratios and 95% CI (forest plots) of clinically relevant adverse event groups associated with renal impairment comparing tiotropium/olodaterol with the monocomponents.
Abbreviations: MACE, major adverse cardiovascular event; Olo, olodaterol; Tio, tiotropium.
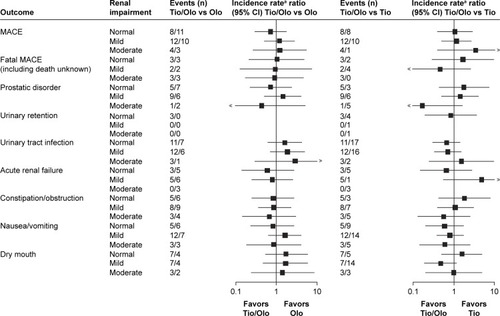
Figure S1 Exposure-adjusted incidence rate ratios and 95% confidence intervals (forest plots) of clinically relevant adverse event groups associated with renal impairment comparing tio/olo with the monocomponents.
Notes: (A) Cardiovascular events. (B) Respiratory events. (C) Urinary tract events. (D) Potential anticholinergic events. aTreatment exposure time adjusted.
Abbreviations: MACE, major adverse cardiovascular event; Olo, olodaterol; Tio, tiotropium.