Figures & data
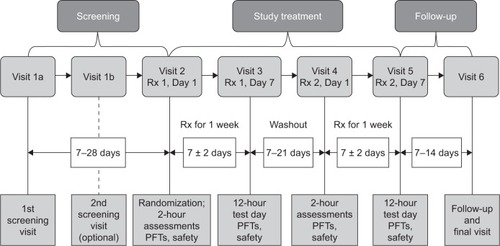
Figure 2 Patient disposition.
Abbreviations: FEV1, forced expiratory volume in 1 second; FF, formoterol fumarate; GFF, glycopyrrolate/formoterol fumarate; GP, glycopyrrolate; MDI, metered dose inhaler.
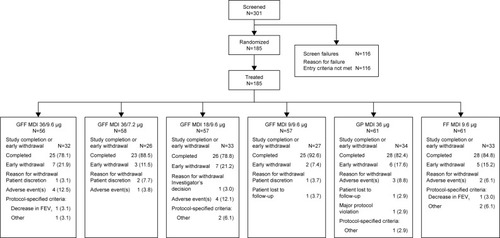
Table 1 Baseline demographics and clinical characteristics (safety population)
Figure 3 (A) FEV1 AUC0–12 on Day 7a,b (B) Change from baseline in FEV1 over time on Day 7c (mITT population).
Abbreviations: AUC0–12, area under the curve from 0 to 12 hours; CI, confidence interval; FEV1, forced expiratory volume in 1 second; FF, formoterol fumarate; GFF, glycopyrrolate/formoterol fumarate; GMR, geometric mean ratio; GP, glycopyrrolate; LSM, least squares mean; MDI, metered dose inhaler; mITT, modified intent-to-treat; N/A, not applicable; SE, standard error.
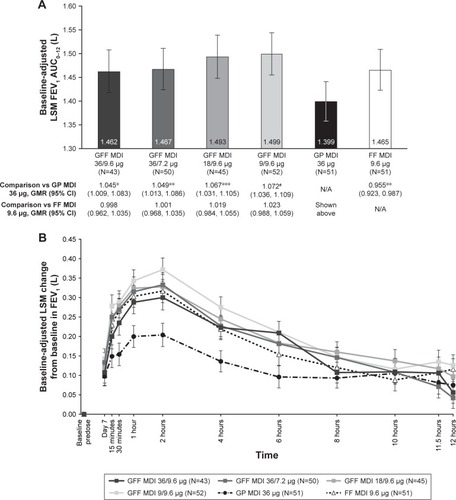
Table 2 Summary of secondary efficacy endpoints on Day 1 (mITT population)
Figure 4 (A) Peak change from baseline in FEV1 through 2 hours on Day 1a,b (B) Peak change from baseline in IC on Day 1a,b (mITT population).
Abbreviations: CI, confidence interval; FEV1, forced expiratory volume in 1 second; FF, formoterol fumarate; GFF, glycopyrrolate/formoterol fumarate; GP, glycopyrrolate; IC, inspiratory capacity; LSM, least squares mean; MDI, metered dose inhaler; mITT, modified intent-to-treat; N/A, not applicable.
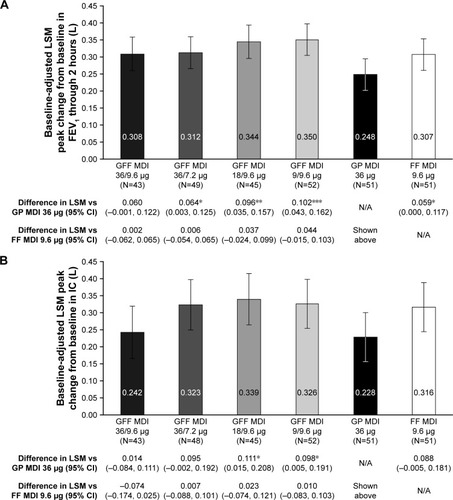
Table 3 Summary of secondary efficacy endpoints on Day 7 (mITT population)
Figure 5 (A) Peak change from baseline in FEV1 through 6 hours on Day 7a,b (B) Peak change from baseline in IC on Day 7a,b (mITT population).
Abbreviations: CI, confidence interval; FEV1, forced expiratory volume in 1 second; FF, formoterol fumarate; GFF, glycopyrrolate/formoterol fumarate; GP, glycopyrrolate; IC, inspiratory capacity; LSM, least squares mean; MDI, metered dose inhaler; mITT, modified intent-to-treat; N/A, not applicable.
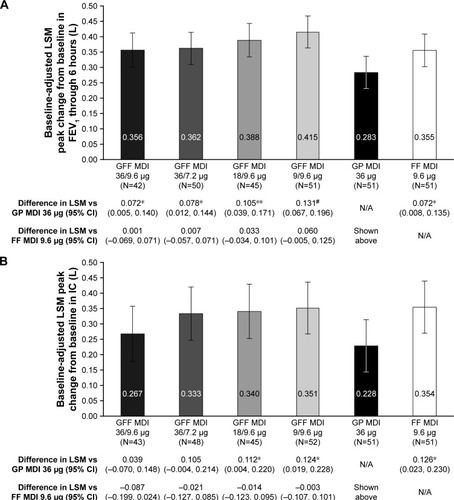
Table 4 Summary of AEs (safety population)