Figures & data
Table 1 Demographics and baseline clinical characteristics for the Japanese subgroup of the DYNAGITO study (treated set)
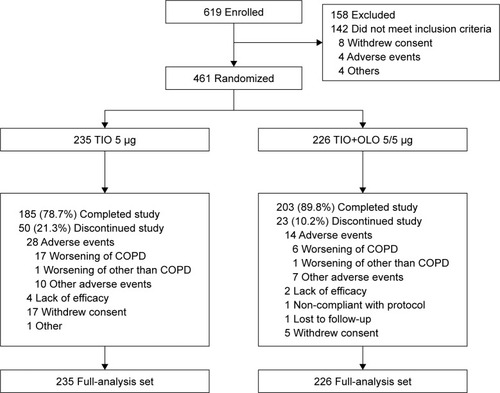
Figure 2 Probability of treatment discontinuation by treatment group.
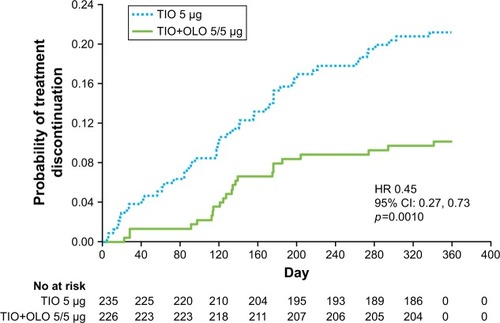
Figure 3 Annualized rate of COPD exacerbations by treatment group.
Notes: Error bars represent the 99% CI for rate of moderate-to-severe COPD exacerbations and the 95% CI for rate of severe COPD exacerbations. Listed values are RR (CI: *99% CI; ^95% CI), p-value. Negative binominal model, adjusted for treatment exposure. The duration of an event is not included in the calculation of a patient’s exposure.
Abbreviations: OLO, olodaterol; TIO, tiotropium; RR, rate ratio.
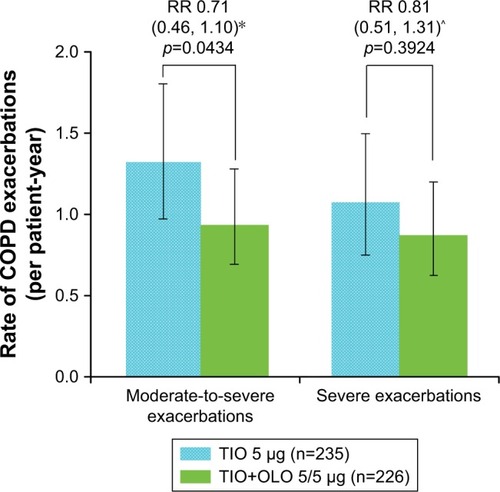
Figure 4 Event rate ratio of moderate-to-severe exacerbations by baseline demographics and pulmonary baseline therapy.
Notes: *RR not available because no incidence was observed in the TIO+OLO arm; ^subgroup division was based on the median SGRQ total score in the trial population at baseline (median=39).
Abbreviations: GOLD, Global Initiative for Chronic Obstructive Lung Disease; ICS, inhaled corticosteroid; LABA, long-acting beta-agonist; LAMA, long-acting muscarinic antagonist; OLO, olodaterol; TIO, tiotropium; SGRQ, St George’s Respiratory Questionnaire; RR, rate ratio.
Figure 5 Cumulative risk of first moderate-to-severe COPD exacerbation by treatment group.
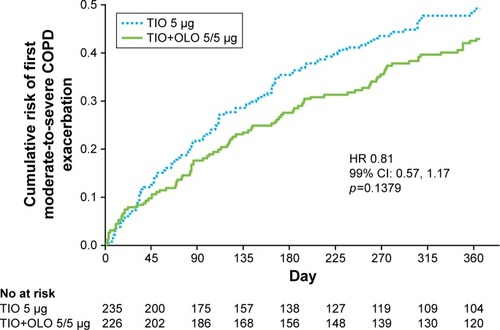
Figure 6 Annualized rate of COPD exacerbations requiring antibiotics, systemic corticosteroids, antibiotics + systemic corticosteroids, and hospitalization.
Abbreviations: OLO, olodaterol; TIO, tiotropium; RR, rate ratios.
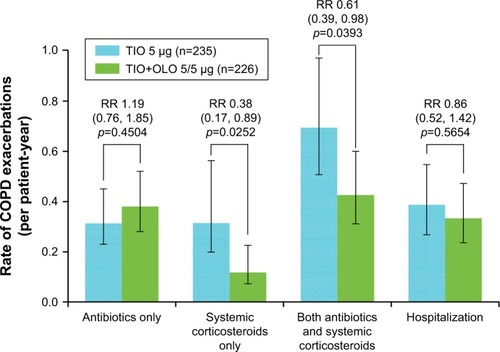
Table 2 Summary of AEs by preferred terms (safety set)