Figures & data
Table 1 Primer sets
Table 2 Retention times of unknown nucleosides and their concentrations in Ophiocordyceps sinensis
Figure 1 Typical chromatograms of (A) nucleoside standards and (B) unknown peaks in C. sinensis determined by high-performance liquid chromatography.
Abbreviation: C. sinensis, Ophiocordyceps sinensis.
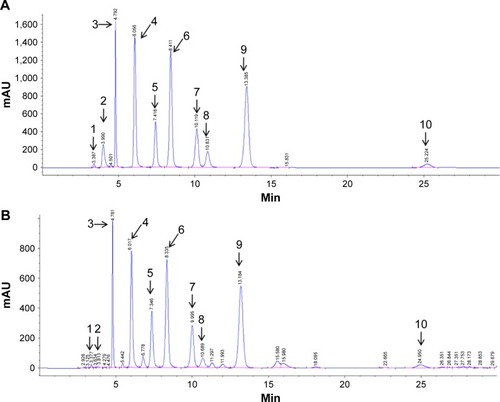
Figure 2 Effects of the isolated nucleosides on NO and iNOS expression in RAW264.7 cells.
Abbreviations: CSE, cigarette smoke extract; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; iNOS, inducible nitric oxide synthase; NO, nitric oxide.
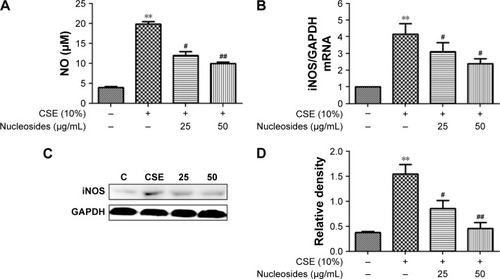
Figure 3 Effects of the nucleosides on CSE-induced TNF-α, IL-6, and IL-1β expression in RAW264.7 cells.
Abbreviations: CSE, cigarette smoke extract; IL-1β, interleukin-1β; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α.
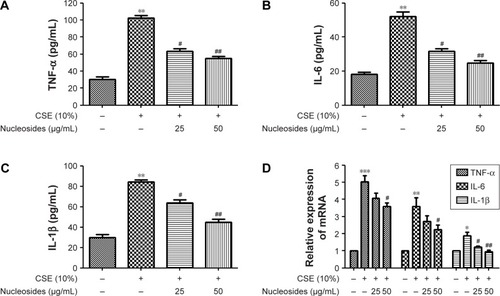
Figure 4 Effects of the nucleosides on (A) mRNA, (B) Western blot, and (C) quantity of SIRT1, as well as (D) Western blot and (E) quantity of NF-κB/p65 in CSE-induced RAW264.7 cells.
Abbreviations: CSE, cigarette smoke extract; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; iNOS, inducible nitric oxide synthase; NF-κB, nuclear factor-κB; NO, nitric oxide; SIRT1, silent information regulator 1.
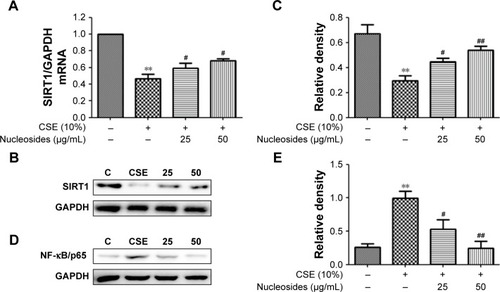
Figure 5 Impact of the SIRT1 antagonist EX527 on treatment effects of the nucleosides on the CSE-induced RAW264.7 cells.
Abbreviations: CSE, cigarette smoke extract; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IL-1β, interleukin-1β; IL-6, interleukin-6; iNOS, inducible nitric oxide synthase; NF-κB, nuclear factor-κB; NO, nitric oxide; SIRT1, silent information regulator 1.
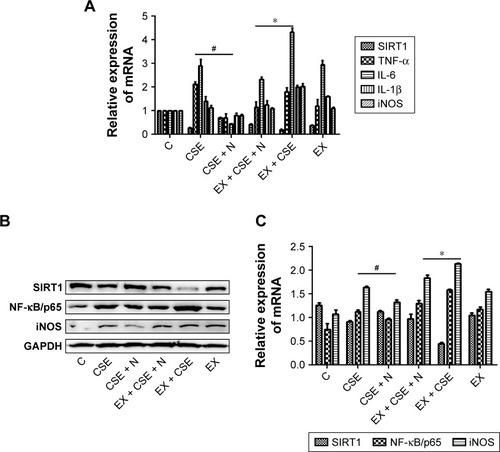
Figure 6 Effects of the nucleosides on (A) NO, (B) TNF-α, (C) IL-1β, and (D) inflammatory cells in CSE-induced mice.
Abbreviations: BALF, bronchoalveolar lavage fluid; CSE, cigarette smoke extract; IL-1β, interleukin-1β; NO, nitric oxide; TNF-α, tumor necrosis factor-α.
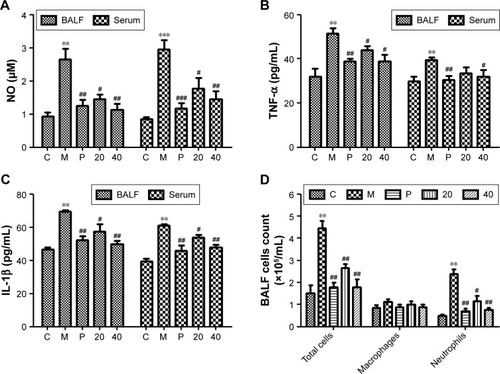
Figure 7 Effects of the nucleosides on lung histological changes measured by H&E staining and the associated protein expression by immunohistochemistry and Western blot analysis.
Abbreviations: CSE, cigarette smoke extract; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; iNOS, inducible nitric oxide synthase; NF-κB, nuclear factor-κB; SIRT1, silent information regulator 1.
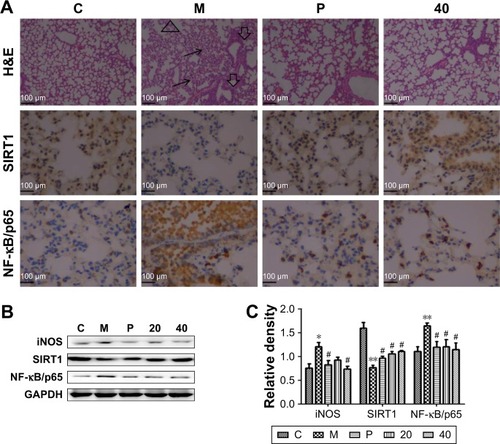
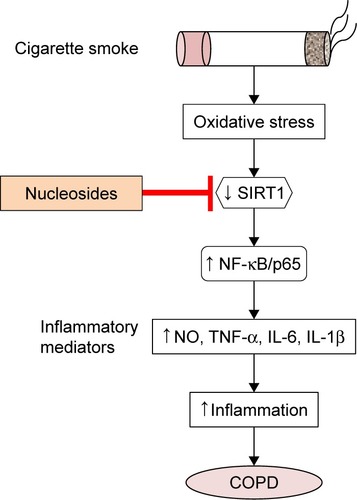
Figure 8 Proposed therapeutic mechanism of the nucleosides on cigarette smoke extract-induced inflammation via the SIRT1–NF-κB/p65 pathway.