Figures & data
Table 1 Study characteristics of the included trials in NMA
Table 2 Baseline characteristics and risk of bias of the included trials in NMA
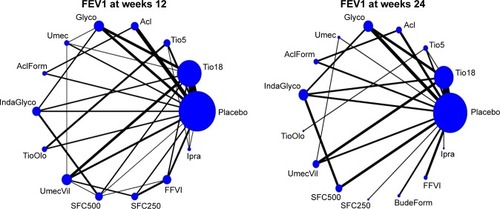
Table 3 Treatment effects on FEV1 at week 12 – NMA results: combining direct and indirect evidence (lower triangle) and direct evidence (upper triangle)
Table 4 Treatment effects on FEV1 at week 24 – NMA results: combining direct and indirect evidence (lower triangle) and direct evidence (upper triangle)
Table 5 SUCRA values for all interventions for each outcome