Figures & data
Table 1 Demographic characteristics (safety population)
Table 2 CHF6001 derived pharmacokinetic parameters following single doses (PK population)
Figure 1 CHF6001 plasma pharmacokinetic–time profile following single doses (PK population).
Abbreviations: PK, pharmacokinetics; MDDPI, study medication administered via multi-dose dry-powder inhaler; SDDPI, study medication administered via single-dose dry-powder inhaler.
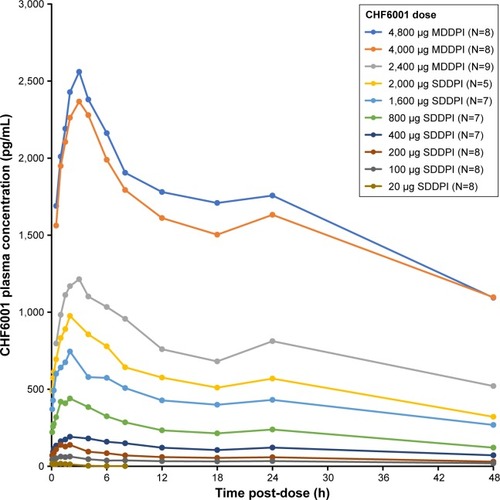
Table 3 CHF6001 derived pharmacokinetic parameters following OD or BID administration (PK population)
Figure 2 CHF6001 plasma pharmacokinetic–time profile following multiple OD administration via SDDPI (PK population).
Notes: Data are mean. PK population is defined as all subjects in the safety population who had at least one valid PK measurement and who had no major PK-related protocol deviations.
Abbreviations: PK, pharmacokinetics; OD, once daily; SDDPI, study medication administered via single-dose dry-powder inhaler.
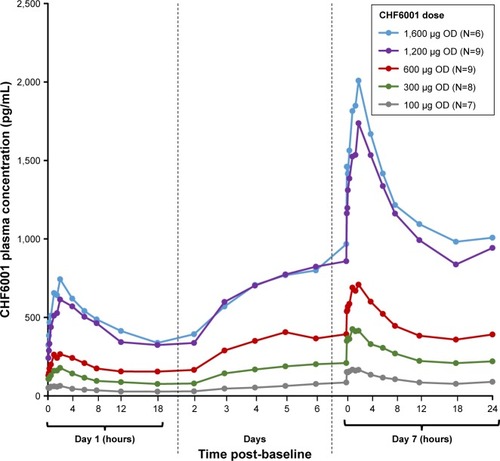
Figure 3 CHF6001 plasma pharmacokinetic–time profile following multiple BID administration via MDDPI (PK population).
Abbreviations: BID, twice daily; MDDPI, study medication administered via multi-dose dry-powder inhaler; PK, pharmacokinetics.
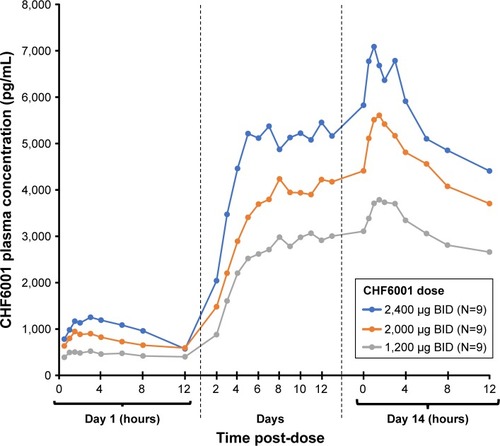
Table 4 CHF6001 derived pharmacokinetic parameters following OD or BID administration via MDDPI
Figure 4 Plasma concentration of CHF6001 following BID administration via MDDPI: simulation of data from 100 subjects replicated 10 times.
Abbreviations: BID, twice daily; MDDPI, study medication administered via multi-dose dry-powder inhaler.
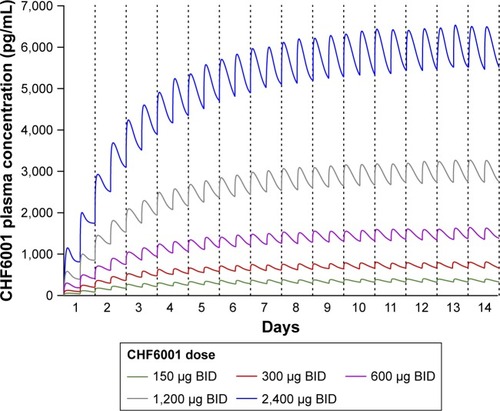
Figure 5 Plasma concentration of CHF6001 following OD or BID administration of the same total daily dose via MDDPI: simulation of data from 100 subjects replicated 10 times.
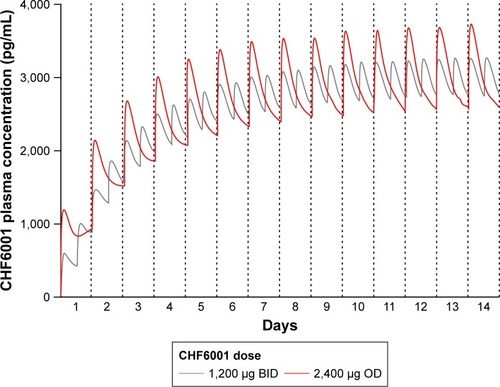
Table 5 Number (%) of subjects reporting AEs and SAEs following administration of single doses (safety population)
Table 6 Number (%) of subjects reporting AEs and SAEs following administration of multiple doses (safety population)
Data availability
The data of this study are available on request, following submission of a valid research protocol to the corresponding author.
