Figures & data
Table 1 Summary of five articles from PubMed and EMBASE that were determined eligible using the keywords “COPD”, “eosinophil”, and “clinical trial” based on systematic review criteria
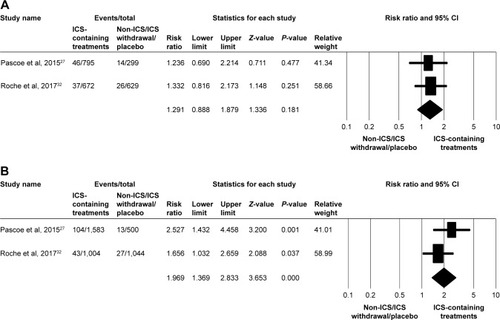
Figure 1 Forest plots of studies comparing the pooled risk ratio for moderate/severe exacerbation in patients with COPD receiving ICS-containing treatment or non-ICS/ICS withdrawal/placebo treatments by subgroup.
Note: (A) Eosinophil counts <2% and (B) eosinophil counts ≥2%.
Abbreviation: ICS, inhaled corticosteroid.
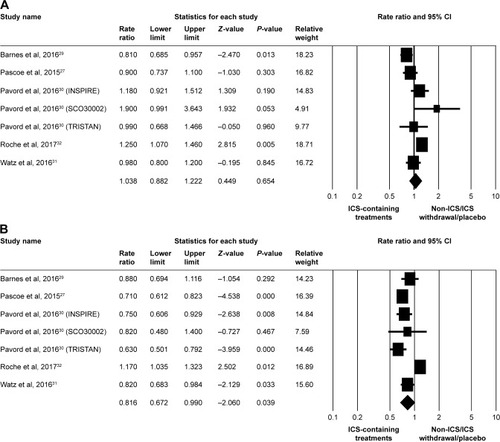
Figure 2 Analysis of publication bias for pooled risk ratio of moderate/severe exacerbation for (A) eosinophil counts <2% and (B) eosinophil counts ≥2%.
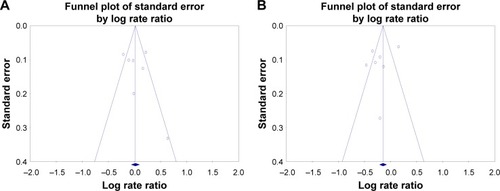
Figure 3 Forest plots of studies comparing the pooled hazard ratio for time-to-first moderate/severe exacerbation in patients with COPD receiving ICS-containing treatment or non-ICS/ICS withdrawal/placebo treatments by subgroup.
Note: (A) Eosinophil counts <2% and (B) eosinophil counts ≥2%.
Abbreviation: ICS, inhaled corticosteroid.
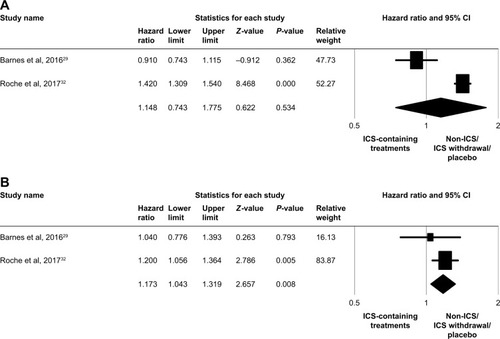
Figure 4 Forest plots of studies comparing the pooled relative risk of pneumonia events in patients with COPD receiving ICS-containing treatment or non-ICS/ICS withdrawal/placebo treatments by subgroup.
Abbreviation: ICS, inhaled corticosteroid.