Figures & data
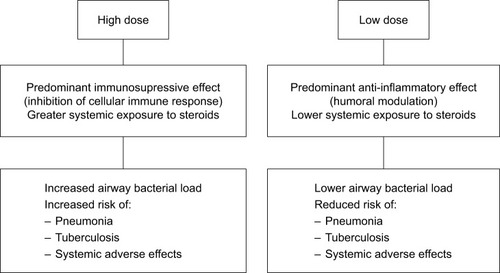
Figure 1 Incidence rates of pneumonia in different studies according to doses of ICS.
Abbreviations: BDP, beclomethasone dipropionate; FF, fluticasone furoate; G, glycopyrronium; ICS, inhaled corticosteroids.
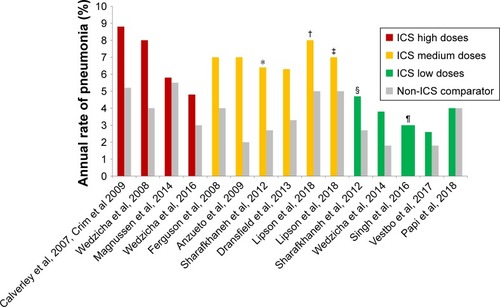
Table 1 Characteristics and results of major long-term Phase III double-blind, randomized controlled clinical trials with ICS in patients with COPD
Figure 2 The dose-response curve of ICS.
Abbreviation: ICS, inhaled corticosteroids.
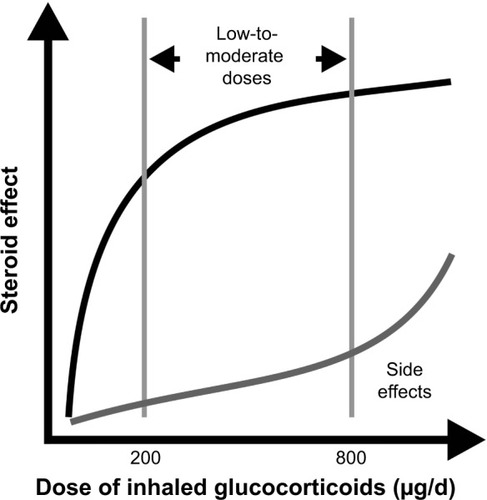
Figure 3 Pathophysiological mechanisms involved in systemic adverse effects of ICS in COPD patients.