Figures & data
Table 1 Baseline characteristics of the intent-to-treat populations in the FORWARD and Dransfield studies
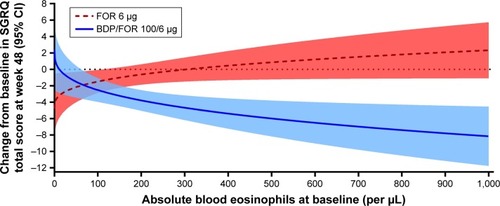
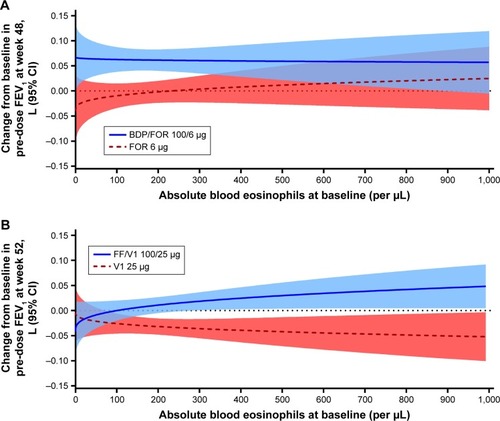
Figure 1 (A) Predicted exacerbation rates by baseline blood eosinophils for the FORWARD study; (B) predicted annual exacerbation rates by baseline blood eosinophils for the Dransfield studies.
Abbreviations: BDP, beclomethasone dipropionate; FF, fluticasone furoate; FOR, formoterol; VI, vilanterol.
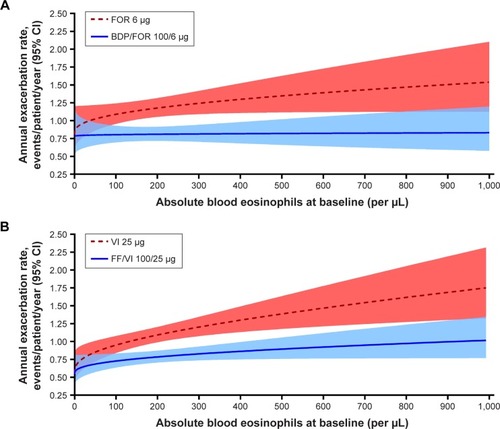
Figure 2 (A) Predicted change from baseline in trough FEV1 by baseline blood eosinophils in the FORWARD study at week 48; (B) predicted change from baseline in trough FEV1 by baseline blood eosinophils in the Dransfield studies at week 52.
Abbreviations: BDP, beclomethasone dipropionate; FF, fluticasone furoate; FOR, formoterol; VI, vilanterol.
Data availability
The manuscript reports results from an analysis of three studies: “FORWARD” NCT0929851 (Chiesi) and replicate Dransfield studies NCT1009463/NCT1017952 (GSK). For GSK clinical trials, within 6 months of publishing the results of the primary endpoints of the study, anonymized individual participant data plus the annotated case report form, protocol, reporting and analysis plan, data set specifications, raw dataset, analysis-ready dataset, and clinical study report are available for research proposals approved by an independent review committee. Proposals should be submitted to www.clinicalstudydatarequest.com. A data access agreement will be required. From January 1, 2019, Chiesi commits to sharing the anonymized, patient-level data, study-level data, the clinical protocol, and the full clinical study report of the FORWARD study (NCT0929851) with qualified scientific and medical researchers, conducting legitimate research. Chiesi provides access to clinical trial information consistently with the principle of safeguarding commercially confidential information and patient privacy. Fundamental conditions for providing the requested clinical trial data are that qualified researchers agree to sign a Data Sharing Agreement, to use the data only for noncommercial purposes and to seek publication of their research results. Other information on Chiesi’s data sharing commitment, access and research request’s approval process will be available from January 1, 2019, in the Clinical Trial Transparency section of this webpage page: http://www.chiesi.com/en/research-and-development/.