Figures & data
Figure 1 Schematic of the frequent exacerbator phenotype.
Abbreviations: CRP, C-reactive protein; FEV1, forced expiratory volume in 1 second; IL-6, interleukin-6.
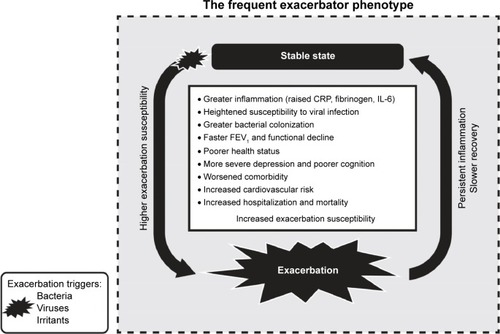
Table 1 Randomized double-blind trials evaluating roflumilast in patients with COPD
Figure 2 Effect of roflumilast on the mean rate of moderate or severe exacerbations with or without a LABA.
Abbreviations: CI, confidence interval; LABA, long-acting β2-agonist.
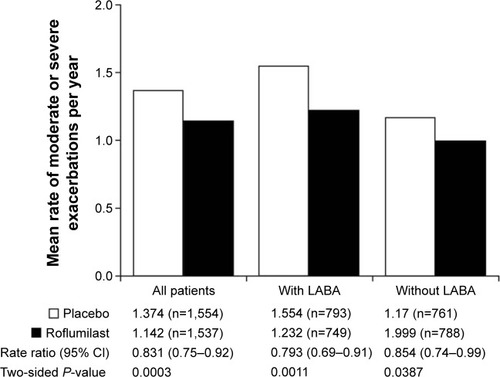
Figure 3 Mean rate of serious exacerbations or exacerbations leading to hospital admission per patient per year in the REACT study.
Abbreviation: CI, confidence interval.
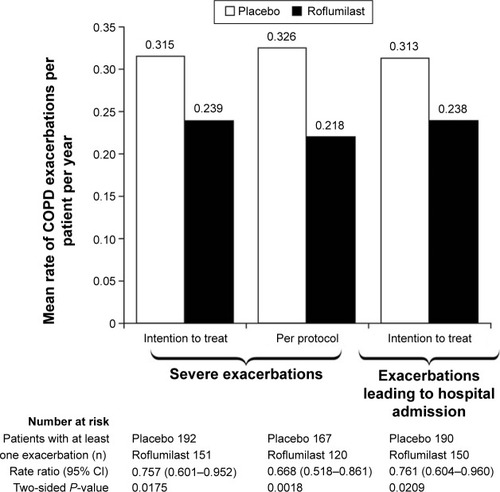
Table 2 Adverse reactions (≥2%) associated with roflumilast from four 1-year placebo-controlled trials and four 6-month trials
Figure 4 Changes in mean glycated hemoglobin (HbA1c) levels over 12 weeks in patients with newly diagnosed, treatment-naïve type 2 diabetes mellitus.
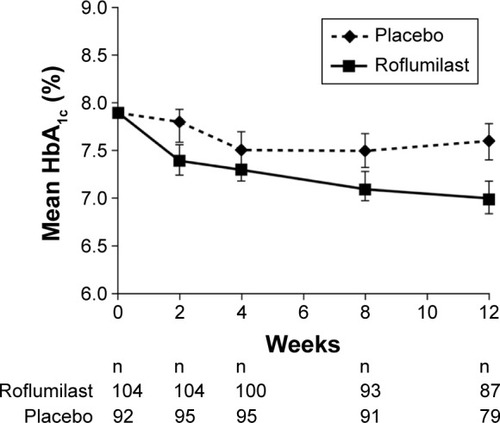
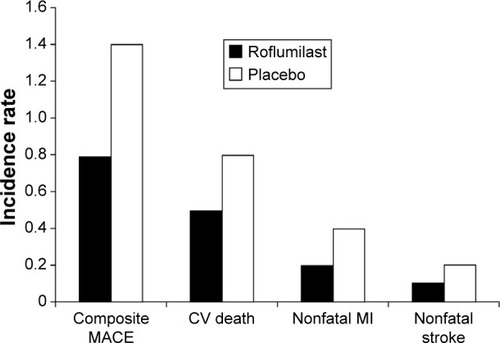
Figure 5 Pooled analysis of the incidence rate of the composite of MACE (nonfatal MI, nonfatal stroke, and CV death) for patients receiving roflumilast (n=6,563) or placebo (n=5,491).
Abbreviations: CV, cardiovascular; MACE, major adverse cardiovascular events; MI, myocardial infarction.