Figures & data
Table 1 Completed Phase III studies comparing clinical effects of tiotropium–olodaterol fixed-dose combinations with monocomponents and/or placebo in patients with COPD
Table 2 Differences in TIO/OLO 5/5 μg, TIO monotherapy, OLO monotherapy, and PLA for different spirometric end points derived from key clinical studies
Table 3 Differences in TIO/OLO 5/5 μg, TIO monotherapy, OLO monotherapy, and PLA in key clinical studies, highlighting number of responders and number needed to treat
Figure 1 Adjusted mean FRC (A) and RV (B) responses at 6 weeks ± SE, measured by body plethysmography at 2 hours 30 minutes (02:30) and 22 hours 30 minutes postdose.
Abbreviations: FRC, functional residual capacity; RV, residual volume; SE, standard error; OLO, olodaterol; TIO, tiotropium; FDC, fixed-dose combination.
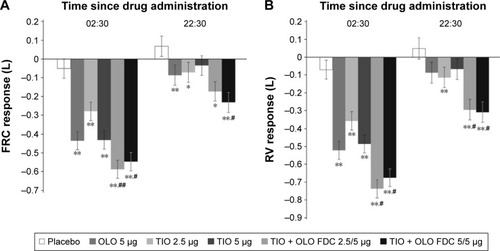
Table 4 Differences between several LAMA/LABA fixed-dose combinations and PLA
