Figures & data
Table 1 Demographic and clinical characteristics of patients who received budesonide/formoterol, formoterol alone, or placebo in the 0–2-month responder analysis, and budesonide/formoterol or formoterol alone in the 12-month response and 2–12-month exacerbation rate analysesTable Footnotea
Table 2 Demographic and clinical characteristics for all patients who received budesonide/formoterol, formoterol, or placebo, and by treatment group, in the two placebo-controlled trials included in the 0–2-month analysis
Table 3 Analysis of 2-month response rates in FEV1 and SGRQ total score in patients who received budesonide/formoterol, formoterol alone, or placebo in the two placebo-controlled trials
Figure 1 Cumulative distribution of (A) FEV1 response and (B) SGRQ response at 2 months in patients who received budesonide/formoterol, formoterol alone, or placebo, by treatment, according to multiple cut-points.
Abbreviations: BUD, budesonide; FEV1, forced expiratory volume in 1 second; FORM, formoterol; SGRQ, St George’s Respiratory Questionnaire.
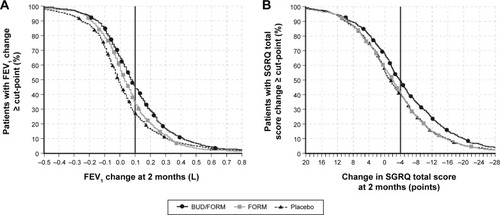
Table 4 Poisson regression analysis of exacerbation rates, by treatment, during months 2–12 in patients who received budesonide/formoterol or formoterol and who did and did not achieve FEV1 and SGRQ response at 2 months
Figure 2 Exacerbation rates during months 2–12 in patients who received budesonide/formoterol or formoterol alone and who achieved (A) FEV1 response and (B) SGRQ improvements above various thresholds at 2 months.
Abbreviations: BUD, budesonide; FEV1, forced expiratory volume in 1 second; FORM, formoterol; SGRQ, St George’s Respiratory Questionnaire.
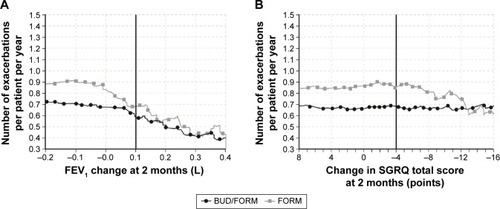
Table 5 Poisson regression analysis of exacerbation rate from 2 to 12 months according to treatment type, baseline FEV1, and SGRQ total score, and FEV1 and SGRQ response at 2 months in patients who received budesonide/formoterol or formoterol
