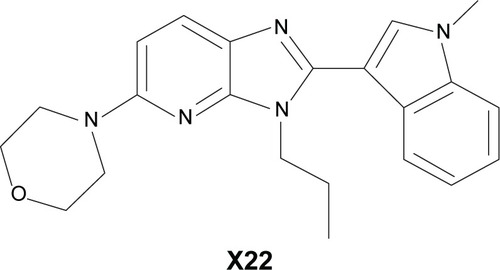
Figures & data
Figure 2 Effect of X22 on MPMs and RAW 264.7 macrophage viability analyzed by an MTT assay.
Abbreviations: DMSO, dimethyl sulfoxide; MPMs, mouse peritoneal macrophages; SEM, standard error of mean.
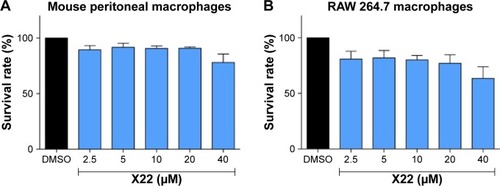
Figure 3 X22 inhibited LPS-induced inflammatory cytokine expression in vitro.
Abbreviations: COX-2, cyclooxygenase-2; DMSO, dimethyl sulfoxide; ELISA, enzyme-linked immunosorbent assay; IL-6, interleukin-6; IL-1β, interleukin-1β; LPS, lipopolysaccharide; qPCR, quantitative polymerase chain reaction; TNF-α, tumor necrosis factor-α; MPMs, mouse peritoneal macrophages; SEM, standard error of mean.
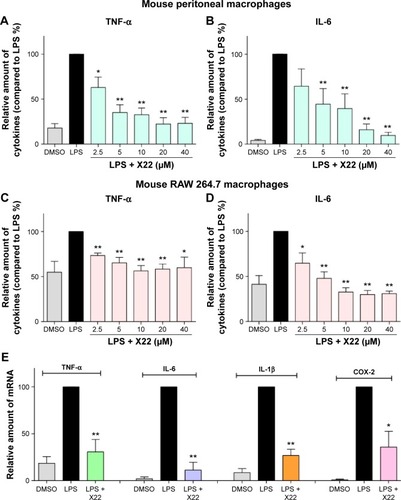
Figure 4 X22 attenuated LPS-induced septic shock in vivo.
Abbreviations: CON, control; ICR mice, Institute of Cancer Research mice; LPS, lipopolysaccharide.
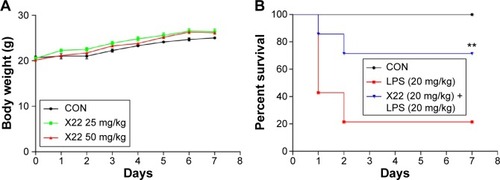
Figure 5 X22 decreased TNF-α expression in the serum of septic mice.
Abbreviations: CON, control; ELISA, enzyme-linked immunosorbent assay; LPS, lipopolysaccharide; TNF-α, tumor necrosis factor-α.
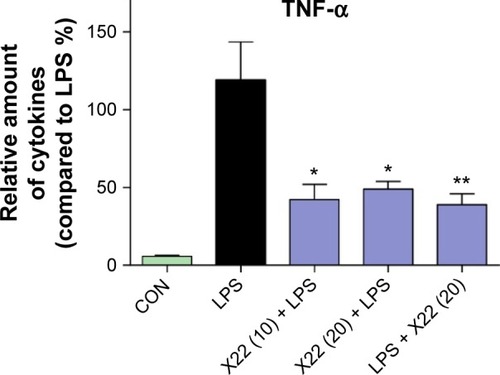
Figure 6 X22 attenuated sepsis-induced lung injury in mice.
Abbreviations: CON, control; H&E staining, hematoxylin and eosin staining; IL-6, interleukin-6; IL-1β, interleukin-1β; iNOS, inducible nitric oxide synthase; LPS, lipopolysaccharide; qPCR, quantitative polymerase chain reaction; TNF-α, tumor necrosis factor-α.
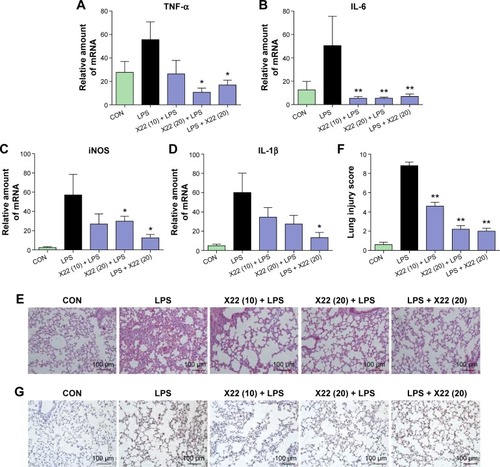
Figure 7 X22 attenuated sepsis-induced liver injury in mice.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate transaminase; CON, control; H&E staining, hematoxylin and eosin staining; IL-6, interleukin-6; IL-1β, interleukin-1β; LPS, lipopolysaccharide; iNOS, inducible nitric oxide synthase; qPCR, quantitative polymerase chain reaction; TNF-α, tumor necrosis factor-α.
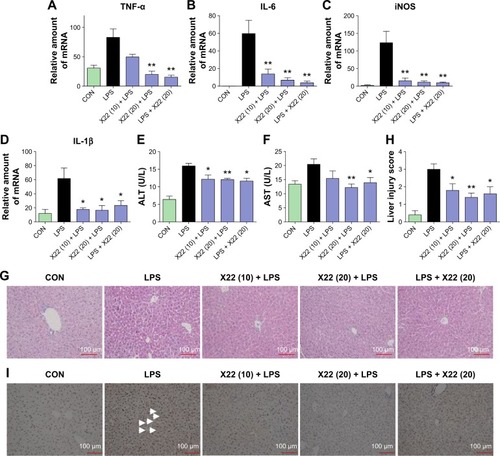
Figure 8 Effect of X22 on LPS-induced acute lung injury.
Abbreviations: BALF, bronchoalveolar lavage fluid; CON, control; COX-2, cyclooxygenase-2; H&E staining, hematoxylin and eosin staining; IL-6, interleukin-6; IL-1β, interleukin-1β; LPS, lipopolysaccharide; qPCR, quantitative polymerase chain reaction; TNF-α, tumor necrosis factor-α.
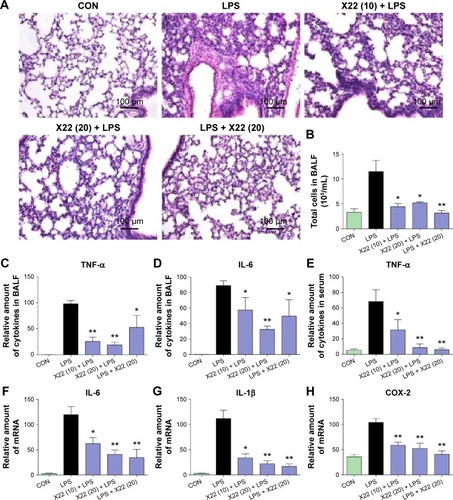
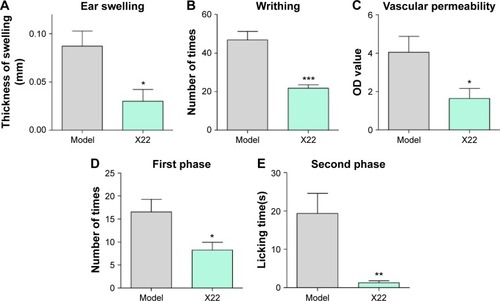
Figure 9 Effects of X22 on chemically-induced inflammation in vivo.
Abbreviations: LPS, lipopolysaccharide; OD value, optical density value; SEM, standard error of mean.