Figures & data
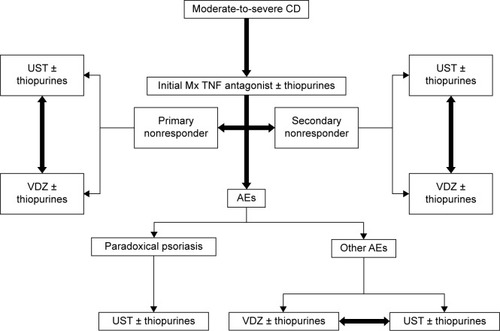
Figure 1 Ustekinumab binds to the p40 subunit of IL-12 and IL-23, preventing binding with the NK- or T-cell surface IL-12Rβ1, and inhibiting IL-12 signaling and further activation of Th1 subset of T cells as well as IL-23 signaling and further activation of Th17 subset of T cells.
Abbreviations: IL, interleukin; IL-12Rβ1, IL-12 receptor β1; NK, natural killer.
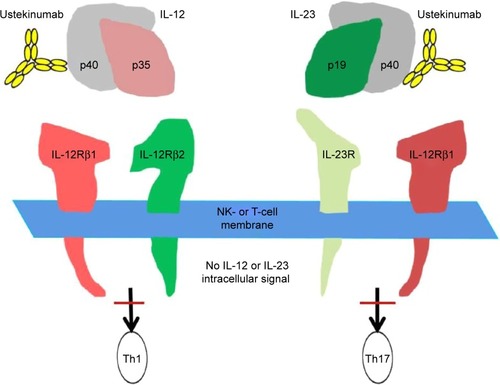
Table 1 Summary of RCTs on ustekinumab in Crohn’s disease
Figure 2 Overall structure of UNITI phase III program.
Abbreviations: LTE, long-term extension; TNF, tumor necrosis factor; UST, ustekinumab; IV, intravenous; SC, subcutaneous; q8 wks, every 8 weeks; q12 wks, every 12 weeks.
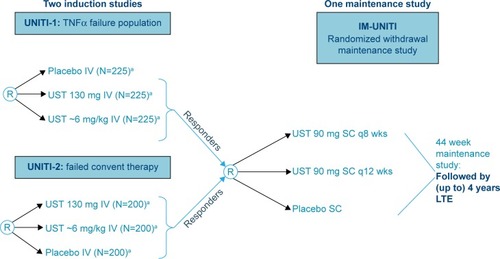
Figure 3 Proposed algorithm for use of UST in moderate-to-severe Crohn’s disease.