Figures & data
Figure 1 Change in PVR from baseline to week 17, per protocol analysis.
Abbreviations: PVR, pulmonary vascular resistance; TE, treatment effect; CL, confi dence limit.
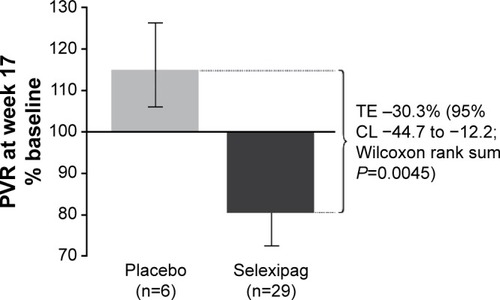
Figure 2 In the GRIPHON clinical trial, the primary composite endpoint included all-cause mortality, disease progression, or worsening of PAH resulting in hospitalization, initiation of parental prostanoid therapy, or long-term oxygen therapy or the need for lung transplantation or atrial septostomy.
Abbreviation: PAH, pulmonary arterial hypertension.
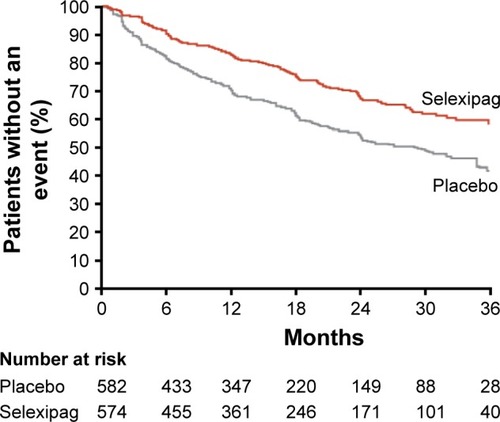
Table 1 Adverse events reported during the GRIPHON clinical trial
Figure 3 Mean (standard deviation) plasma concentration versus time profiles of selexipag and its metabolite ACT-333679 in healthy subjects.
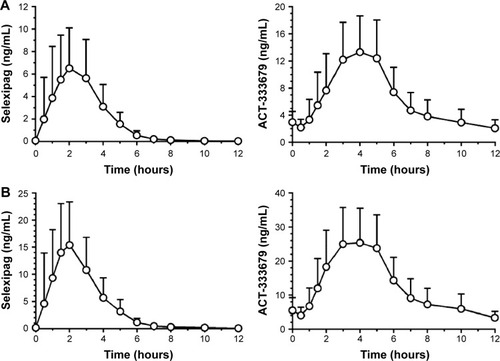
Table 2 Clinical trials evaluating oral prostanoid and nonprostanoid IP receptor agonists in the treatment of PAH
