Figures & data
Figure 1 Effects of UA on neurological deficit scores and the infarct volume.
Abbreviations: UA, ursolic acid; BADGE, bisphenol A diglycidyl ether; TTC, 2,3,5-triphenyltetrazolium chloride.
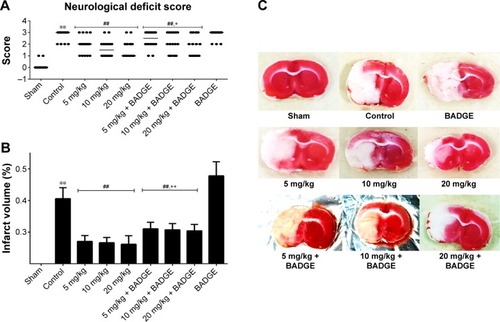
Figure 2 Effect of UAtreatment on brain damage in rats.
Abbreviations: UA, ursolic acid; BADGE, bisphenol A diglycidyl ether; MCAO/R, middle cerebral artery occlusion and reperfusion.
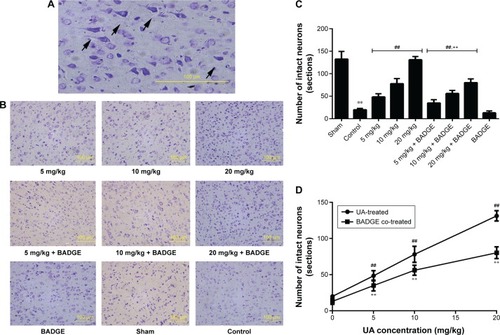
Figure 3 Effect of UA on the PPARγ protein levels.
Abbreviations: UA, ursolic acid; BADGE, bisphenol A diglycidyl ether.
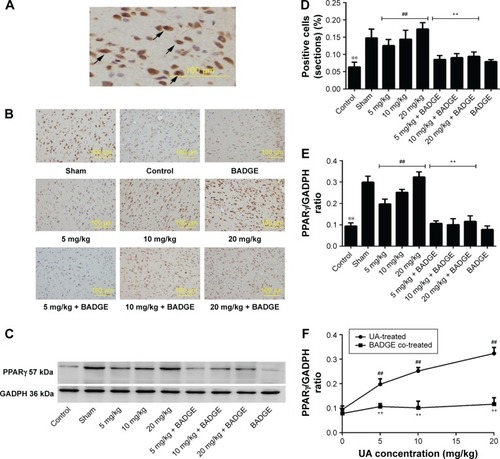
Figure 4 Effects of UA on MMP2 and MMP9.
Abbreviations: UA, ursolic acid; BADGE, bisphenol A diglycidyl ether.
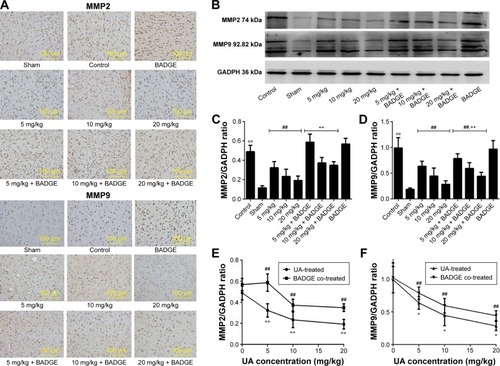
Figure 5 Effect of UA on TIMP1.
Abbreviations: UA, ursolic acid; BADGE, bisphenol A diglycidyl ether.
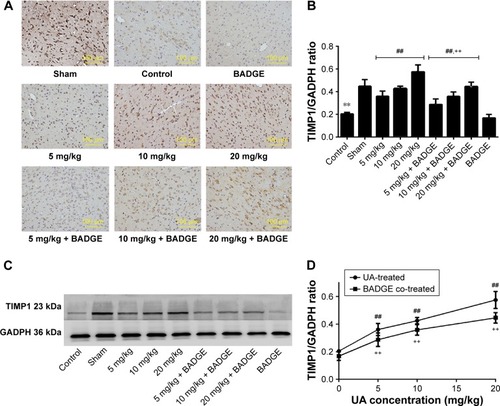
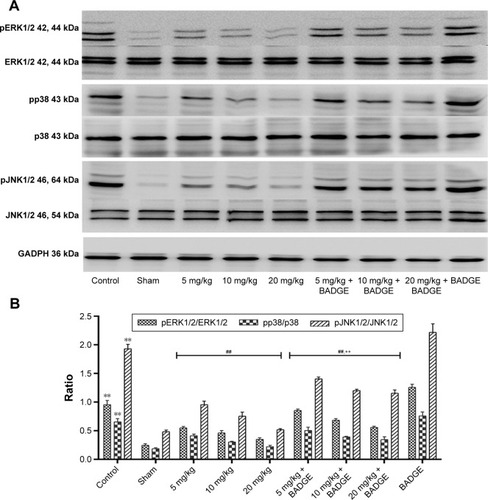
Figure 6 Effect of UA on the MAPK signaling pathway.
Abbreviations: UA, ursolic acid; MAPK, mitogen-activated protein kinase; BADGE, bisphenol A diglycidyl ether.