Figures & data
Table 1 Composition of the probiotics-loaded ECPs-T formulation
Figure 1 Effect of cushioning agents on (A) flow properties of ECP powders; CI and angle of repose (indicated by the red circles), and (B) tablet hardness and disintegration time (indicated by the black circles) in distilled water.
Abbreviations: CI, compressibility index; ECP, enteric-coated pellet; SD, standard deviation; F, formulation.
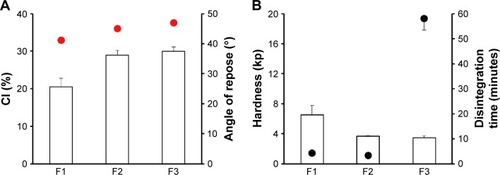
Figure 2 Effect of particle sizes of MCC granules on (A) flow properties of ECP powders; CI and angle of repose (indicated by the red circles), and (B) tablet hardness and disintegration time (indicated by the black circles) in distilled water.
Abbreviations: CI, compressibility index; ECP, enteric-coated pellet; MCC, microcrystalline cellulose; SD, standard deviation; F, formulation.
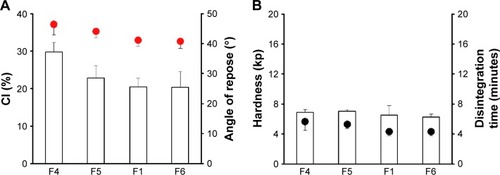
Figure 3 Effect of calcium silicate combined with MCC on (A) flow properties of ECP powders; CI and angle of repose (indicated by the red circles), and (B) tablet hardness and disintegration time (indicated by the black circles) in distilled water.
Abbreviations: CI, compressibility index; ECP, enteric-coated pellet; MCC, microcrystalline cellulose; SD, standard deviation; F, formulation.
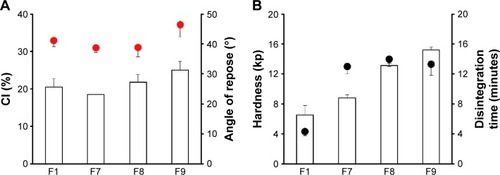
Figure 4 Scanning electron microphotographs of (A) the surface of ECPs and (B) cross-section of ECPs-T.
Abbreviations: ECP, enteric-coated pellet; ECPs-T, ECPs embedded tablet.
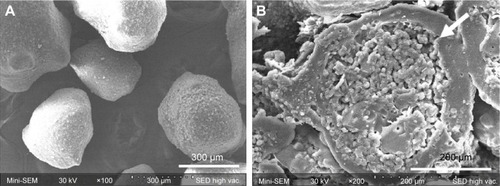
Figure 5 Survival rate of probiotic bacteria during the dry powder coating process and under compression of ECPs into tablet dosage form.
Abbreviations: ECPs, enteric-coated pellets; E. faecalis, Enterococcus faecalis; L. acidophilus, Lactobacillus acidophilus; SD, standard deviation.
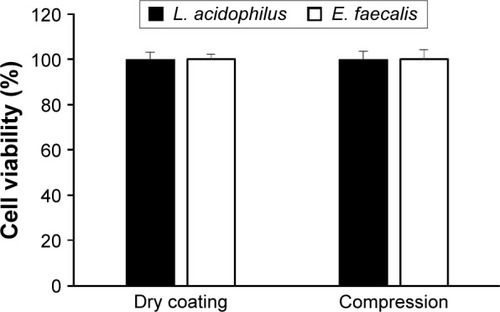
Figure 6 Storage stability of (A) L. acidophilus and (B) E. faecalis in ECPs-T in comparison to corresponding bare bacteria, uncoated probiotic tablet, marketed products, and ECPs under ambient storage conditions (25°C/60% relative humidity) for 6 months.
Abbreviations: ECPs, enteric-coated pellets; ECPs-T, enteric-coated pellets embedded tablet; E. faecalis, Enterococcus faecalis; L. acidophilus, Lactobacillus acidophilus; SD, standard deviation.
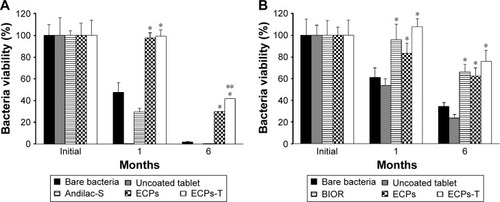
Figure 7 Bacterial viability of (A) L. acidophilus and (B) E. faecalis inside ECPs-T after immersion in acidic medium in comparison to that of free bacteria, uncoated probiotic tablet, marketed products, and ECPs.
Abbreviations: ECPs, enteric-coated pellets; ECPs-T, enteric-coated pellets embedded tablet; E. faecalis, Enterococcus faecalis; L. acidophilus, Lactobacillus acidophilus.
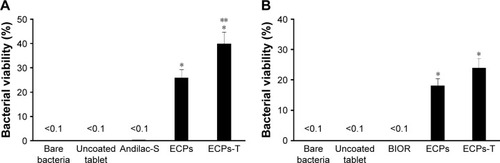
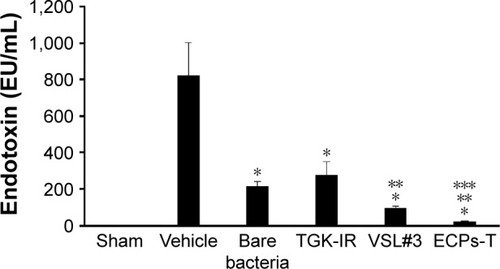
Figure 8 Effect of repeated administration of ECPs-T formulation on plasma endotoxin concentrations in normal rats.
Abbreviations: ECPs-T, enteric-coated pellets embedded tablet; LAL, Limulus amebocyte lysate.