Figures & data
Figure 1 Flow of patients through the study.
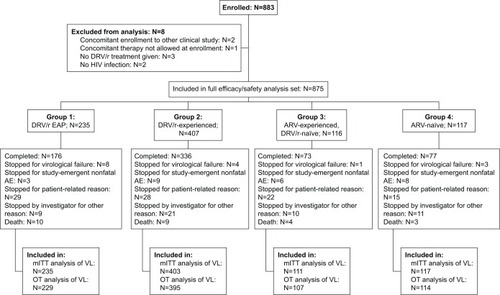
Table 1 Baseline patient characteristics and demographics (full analysis set)
Table 2 Concomitant ARV treatments and DRV/r dose during the study
Table 3 Virological response (VL <50 copies/mL) at last study visit (LOCF) for the mITT and OT analyses in the four HIV-infected patient groups treated with DRV/r, according to VL and CD4+ cell count at study entry
Table 4 Virological response (VL <50 copies/mL) at 48 weeks and 96 weeks for the mITT and OT analyses in the four HIV-infected patient groups treated with DRV/r, according to VL at study entry
Table 5 Reasons for discontinuation from the study
Figure 2 Kaplan–Meier curves from start of prospective observation showing study discontinuation by reason of interruption in Group 1 (n=235) – patients who were part of the DRV/r Early Access Program (EAP).
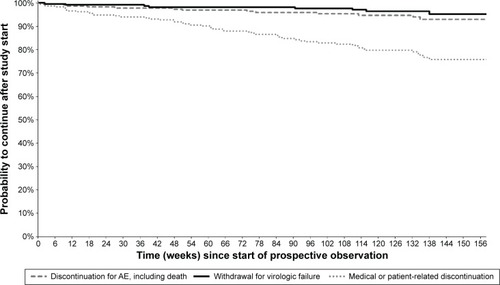
Figure 3 Kaplan–Meier curves from start of prospective observation showing study discontinuation by reason of interruption in Group 2 (n=407) – patients already receiving DRV/r in routine clinical practice.
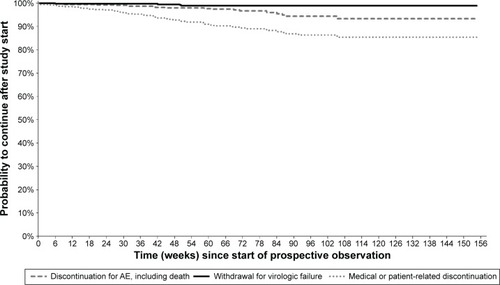
Figure 4 Kaplan–Meier curves from start of prospective observation showing study discontinuation by reason of interruption in Group 3 (n=116) – ARV-experienced DRV-naïve patients.
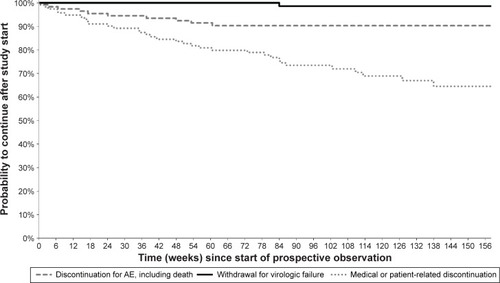
Figure 5 Kaplan–Meier curves from start of prospective observation showing study discontinuation by reason of interruption in Group 4 (n=117) – ARV-naïve patients.
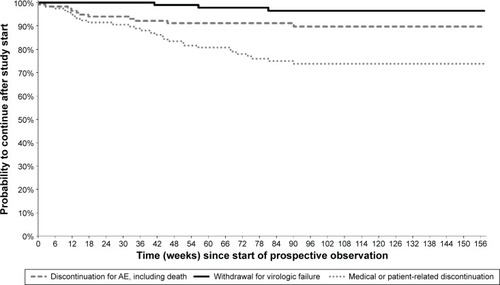
Table 6 CD4+ cell count at study entry and at last study visit in the four groups of HIV-infected patients treated with DRV/r (LOCF analysis)
Table 7 Median (Q1–Q3) of serum biochemistry values during the study in patients with complete observations
