Figures & data
Figure 1 FGF1V–vcMMAE conjugate.
Abbreviations: FGF1V, fibroblast growth factor 1 variant; FGFR, fibroblast growth factor receptor; MMAE, monomethyl auristatin E; vcMMAE, valine–citrulline monomethyl auristatin E.
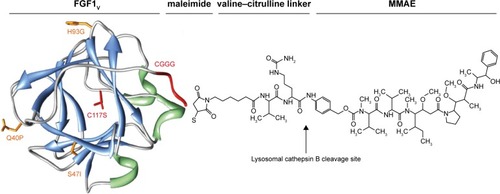
Figure 2 FGF1V–vcMMAE conjugation.
Abbreviations: CR, conjugation reaction sample; FGF1V, fibroblast growth factor 1 variant; M, molecular weight marker; MALDI-MS, matrix-assisted laser desorption-mass spectrometry; SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis; vcMMAE, valine–citrulline monomethyl auristatin E.
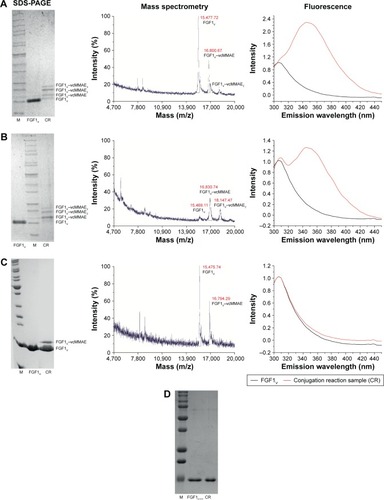
Figure 3 FGF1V–vcMMAE conjugate characteristics.
Abbreviations: FGF1V, fibroblast growth factor 1 variant; FGFR, fibroblast growth factor receptor; vcMMAE, valine–citrulline monomethyl auristatin E.
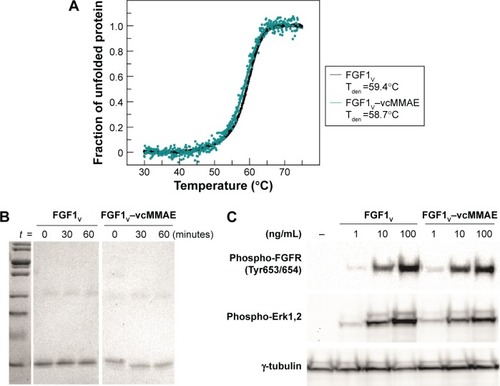
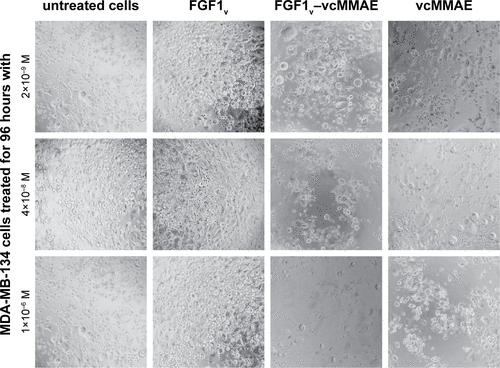
Figure 4 Viability of cells expressing FGFR treated with FGF1V–vcMMAE.
Abbreviations: FGF1V, fibroblast growth factor 1 variant; FGFR, fibroblast growth factor receptor; vcMMAE, valine–citrulline monomethyl auristatin E.
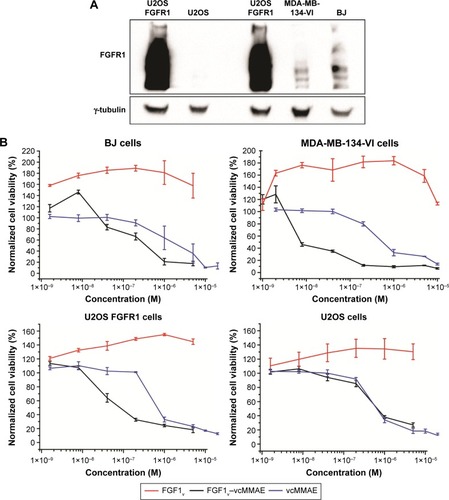
Table 1 Comparison of cytotoxic effects of FGF1V–vcMMAE conjugate and free vcMMAE
Figure 5 Proposed mechanism of action of FGF1V–vcMMAE conjugate.
Abbreviations: FGF1V, fibroblast growth factor 1 variant; FGFR, fibroblast growth factor receptor; MMAE, monomethyl auristatin E; vcMMAE, valine–citrulline monomethyl auristatin E.
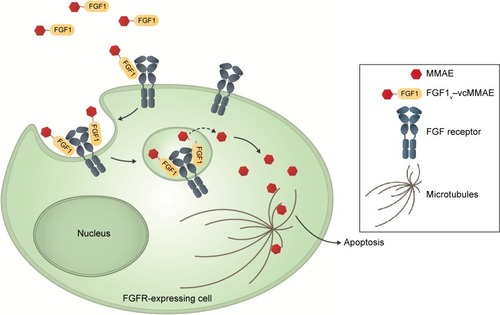
Figure S1 FGFRs mRNA expression levels in studied cell lines.
Abbreviations: FGFR, fibroblast growth factor receptor; mRNA, messenger RNA.
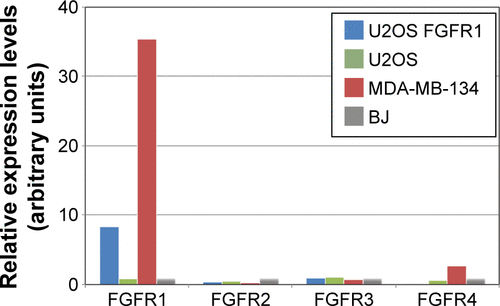
Figure S2 Time-dependency of FGF1V–vcMMAE cytotoxicity.
Abbreviations: FGF1V, fibroblast growth factor 1 variant; vcMMAE, valine–citrulline monomethyl auristatin E.
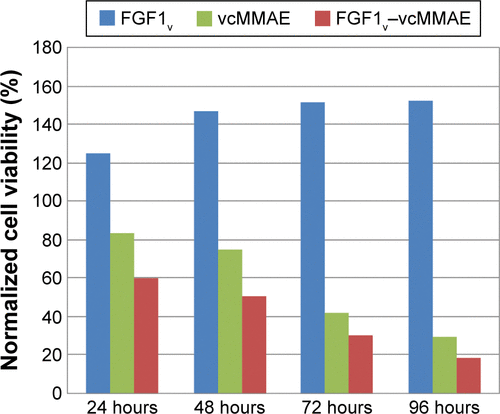
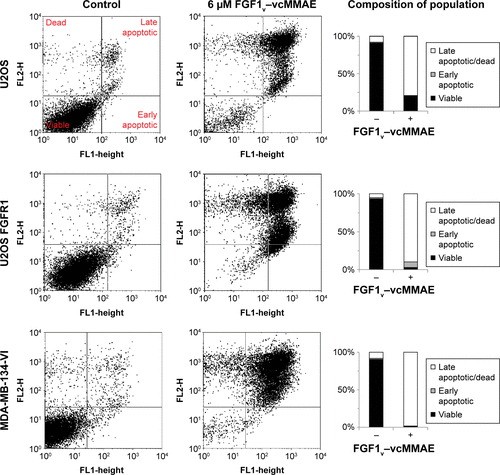
Figure S3 Apoptosis and cell death induced by FGF1V–vcMMAE.
Abbreviations: FGF1V, fibroblast growth factor 1 variant; FGFR, fibroblast growth factor receptor; vcMMAE, valine–citrulline monomethyl auristatin E.