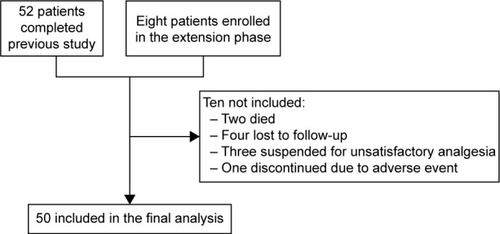
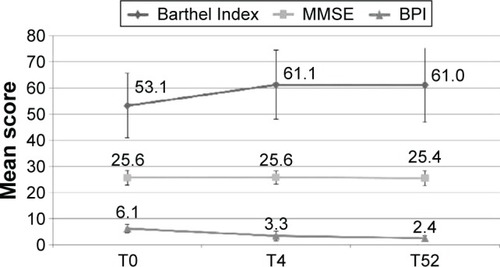
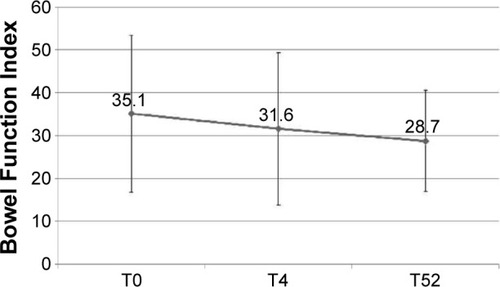
Figures & data
Table 1 Demographic and baseline characteristics of the study population
Table 2 Pain intensity (NRS score) during the 52 weeks of oxycodone/naloxone prolonged-release treatment
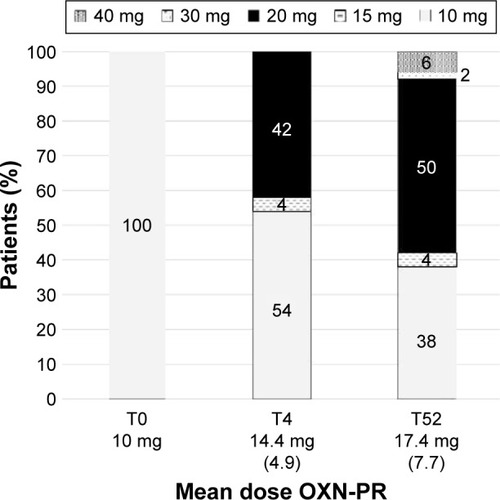
Figure 2 Distribution of OXN-PR daily dosages throughout the observation (expressed in oxycodone equivalents).