Figures & data
Figure 1 The key requirements for the United States Food and Drug Administration.
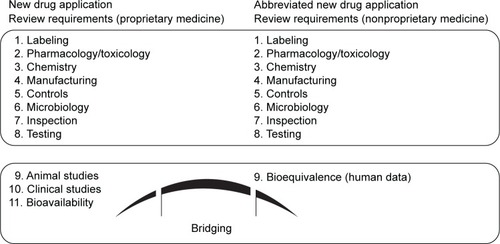
Figure 2 The key differences between proprietary, nonproprietary, substandard, and counterfeit medicines, and substandard copies.
Abbreviation: HA, health authority.
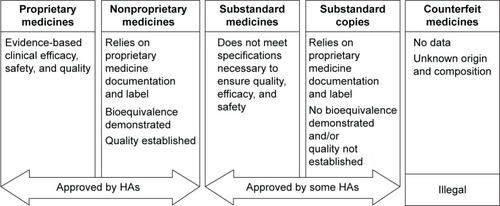
Table 1 Examples of a link between quality parameters and their potential clinical impact
Table 2 Comparison of selected parameters for proprietary versus nonproprietary fingolimod
