Figures & data
Figure 1 Dose–response curves of BJ extract in liver cancer cell lines.
Abbreviations: BJ, Brucea javanica; FITC, fluorescein isothiocyanate; MTT, 3-[4, 5-dimethylthiazol-2-yl]-2, 5 diphenyl tetrazolium bromide; PI, propidium iodide; RPMI, Roswell Park Memorial Institute; SD, standard deviation.
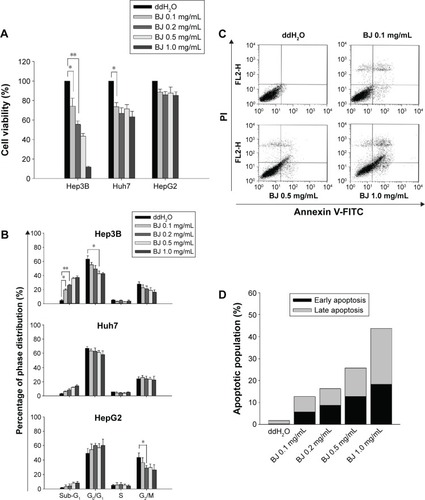
Figure 2 Release of cytochrome c from mitochondria.
Abbreviations: BJ, Brucea javanica; DAPI, 4′,6-diamidino-2-phenylindole; TRITC, tetramethylrhodamine isothiocyanate.
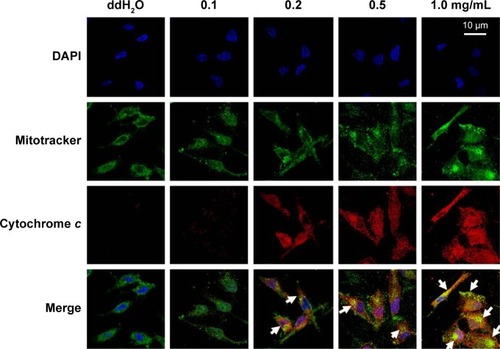
Figure 3 Effects of BJ on apoptotic features in Hep3B cells.
Abbreviations: BJ, Brucea javanica; DMEM, Dulbecco’s Modified Eagle’s Medium; ECL, enhanced chemiluminescence; EGFR, epidermal growth factor receptor; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; PARP, poly(adenosine diphosphate-ribose) polymerase; RPMI, Roswell Park Memorial Institute.
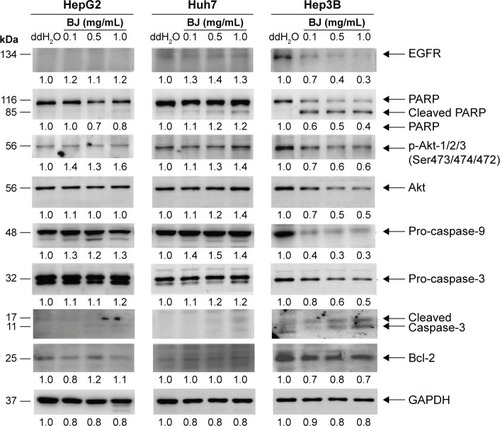
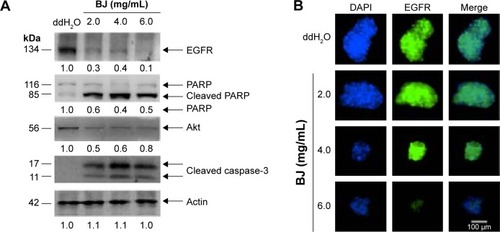
Figure 4 BJ suppressed the growth of Hep3B spheroids.
Abbreviation: BJ, Brucea javanica.
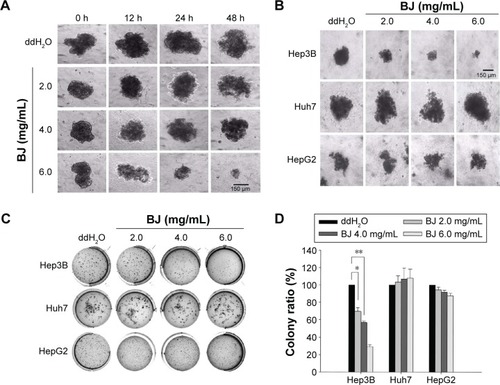
Figure 5 BJ induced apoptotic characteristics in Hep3B spheroids.
Abbreviations: BJ, Brucea javanica; BrdU, bromodeoxyuridine; DAPI, 4′,6-diamidino-2-phenylindole; TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling.
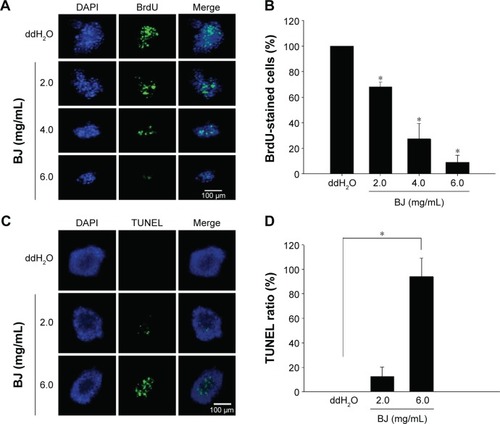
Figure 6 BJ reduced stemness marker of Hep3B spheres.
Abbreviations: BJ, Brucea javanica; DAPI, 4′,6-diamidino-2-phenylindole; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; PAGE, polyacrylamide gel electrophoresis; SDS, sodium dodecyl sulfate.
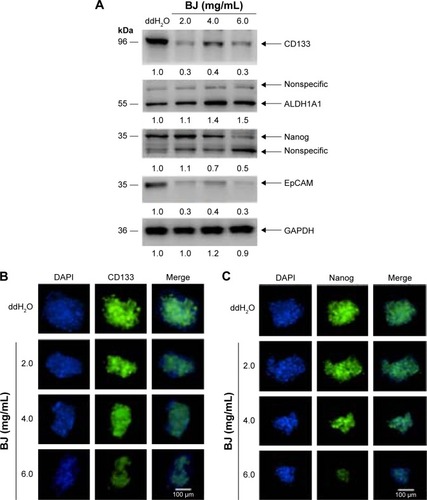
Figure 7 BJ increased apoptotic characteristics of Hep3B spheres.
Abbreviations: BJ, Brucea javanica; DAPI, 4′,6-diamidino-2-phenylindole; EGFR, epidermal growth factor receptor; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; PARP, poly(adenosine diphosphate-ribose) polymerase.