Figures & data
Figure 1 Genesis concept of the novel sulfonylhydrazones 4–9 from molecular modifications based on prototype 3.
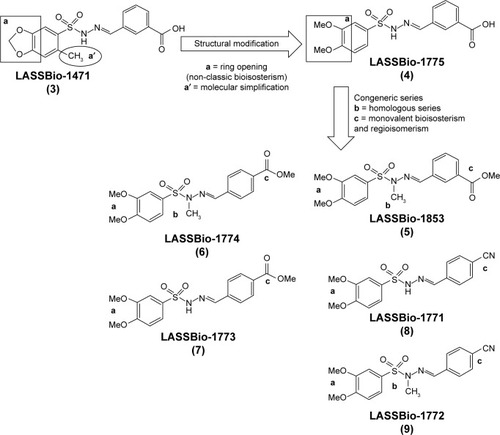
Figure 2 Reagents and conditions.
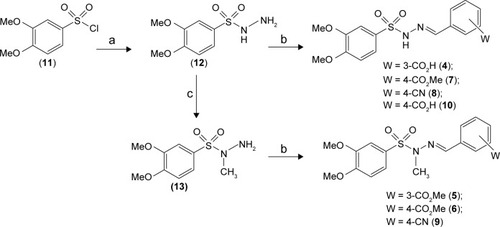
Table 1 Physicochemical properties and aqueous solubility of compounds 4–9
Figure 3 In vitro chemical stability.
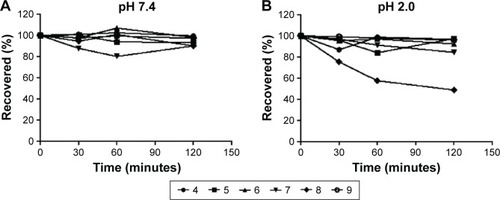
Table 2 In vitro stability of compounds 4–9 in rat plasma
Figure 4 Representative chromatograms of LASSBio-1773 (7) (20 µM) and its metabolite formed by incubation with rat plasma.
Abbreviations: HPLC/MS, high performance liquid chromatography/mass spectrometry; min, minutes; Intens, Intensity.
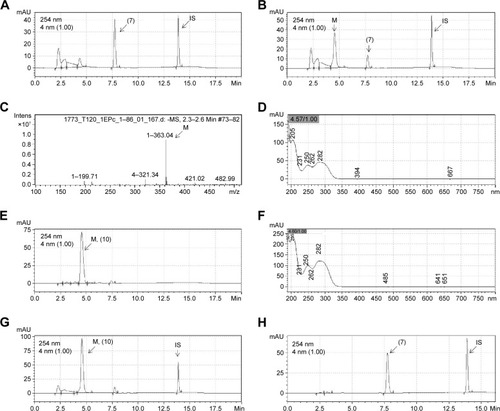
Figure 5 Representative chromatograms of LASSBio-1771 (8) (20 µM) and its metabolite formed by incubation with rat plasma.
Abbreviations: HPLC/MS, high performance liquid chromatography/mass spectrometry; min, minutes; UV, ultraviolet spectroscopy; Intens, Intensity.
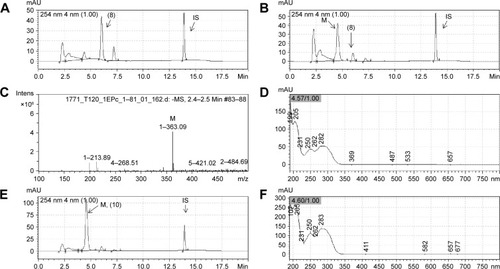
Figure 6 Activity of compounds 4–9 in a murine model of diabetes induced by streptozotocin.
Abbreviation: DMSO, dimethyl sulfoxide.
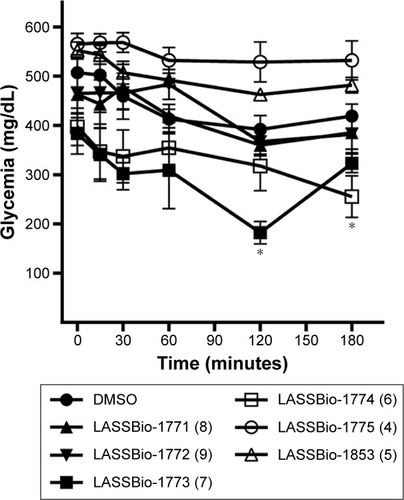
Table 3 Evaluation of blood glucose levels (mg/dL) in diabetic rats treated with vehicle (DMSO) and LASSBio-1773 (7), 50 mg·kg−1, ip for 7 days
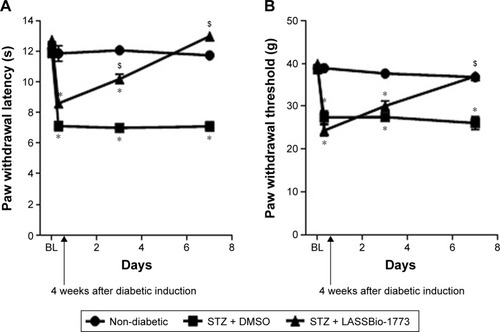
Figure 7 Effect of administration of compound 7 (LASSBio-1773) on diabetic neuropathy.
Abbreviations: DMSO, dimethyl sulfoxide; STZ, streptozotocin; s, seconds.