Figures & data
Table 1 Clinical parameters of EAE
Figure 1 Treatment with moringin ameliorates clinical score in EAE mice.
Abbreviations: EAE, experimental autoimmune encephalomyelitis; SEM, standard error of mean; GMG, glucomoringin; ITC, isothiocyanate.
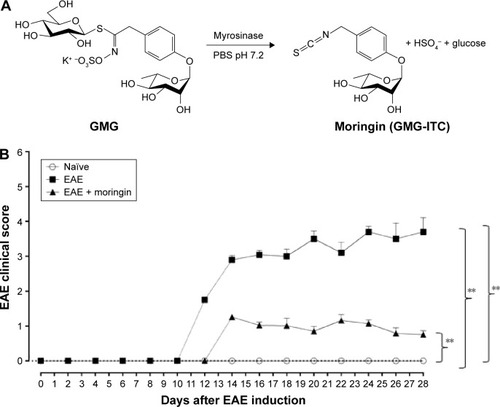
Figure 2 Moringin modulates the Wnt–β-catenin signaling pathway in EAE.
Abbreviations: EAE, experimental autoimmune encephalomyelitis; Ctl, control; ND, not detectable.
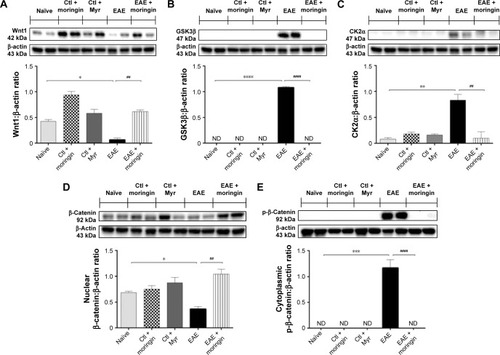
Figure 3 Moringin modulates Fas-ligand expression in EAE.
Abbreviations: EAE, experimental autoimmune encephalomyelitis; Ctl, control.
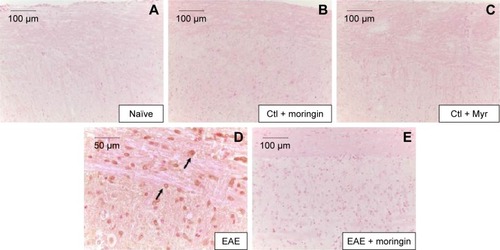
Figure 4 Moringin modulates cleaved caspase-9 expression in EAE.
Abbreviations: EAE, experimental autoimmune encephalomyelitis; Ctl, control.
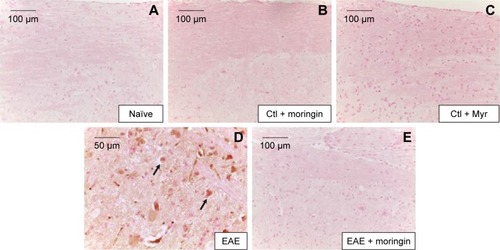
Figure 5 Moringin modulates CD4 expression in EAE.
Abbreviations: EAE, experimental autoimmune encephalomyelitis; Ctl, control.
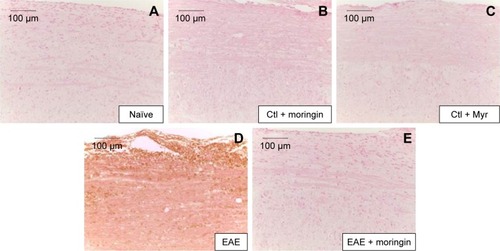
Figure 6 Moringin modulates FoxP3 expression in EAE.
Abbreviations: EAE, experimental autoimmune encephalomyelitis; Ctl, control.
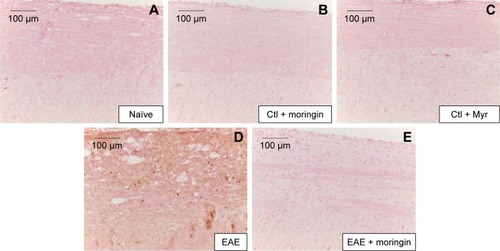
Figure 7 Moringin modulates IL-1β expression in EAE.
Abbreviations: EAE, experimental autoimmune encephalomyelitis; Ctl, control.
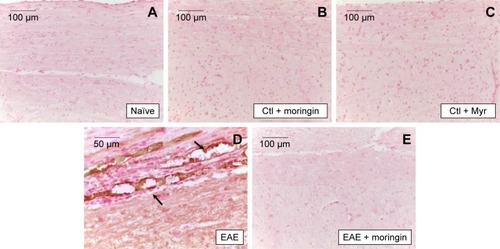
Figure 8 Moringin modulates IL-6 expression in EAE.
Abbreviations: EAE, experimental autoimmune encephalomyelitis; Ctl, control.
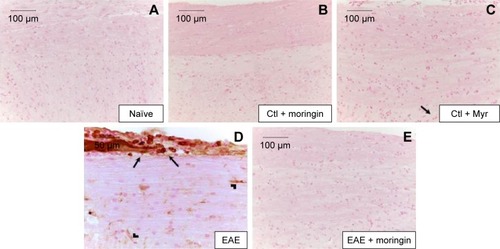
Figure 9 Moringin-modulated inflammatory mediators and Nrf2 activity in EAE.
Abbreviations: EAE, experimental autoimmune encephalomyelitis; Ctl, control; ND, not detectable.
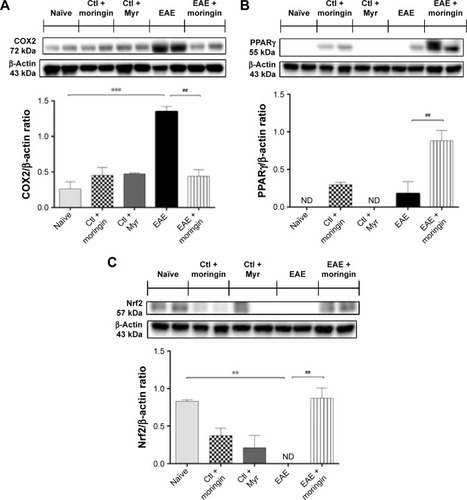
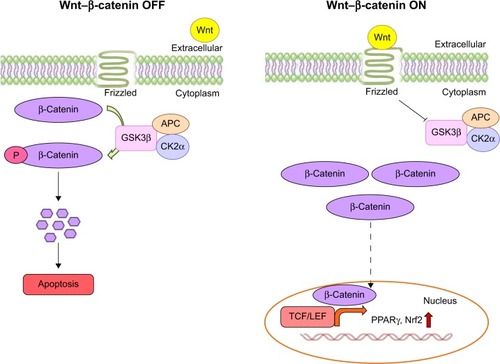
Figure 10 Wnt–β-catenin canonical pathway.