Figures & data
Table 1 Characteristics of 5FU microemulsion
Figure 1 5FU microemulsion image in TEM.
Abbreviations: 5FU, 5-fluorouracil; TEM, transmission electron microscopy.
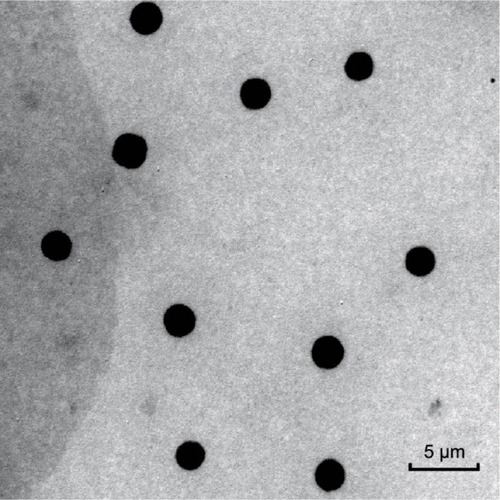
Table 2 Physicochemical properties of the drug-free thermosensitive gel and TG-5FU-ME
Table 3 Stability of 5FU microemulsion
Table 4 Stability of TG-5FU-ME
Figure 2 Appearance of 5FU microemulsion.
Abbreviation: 5FU, 5-fluorouracil.
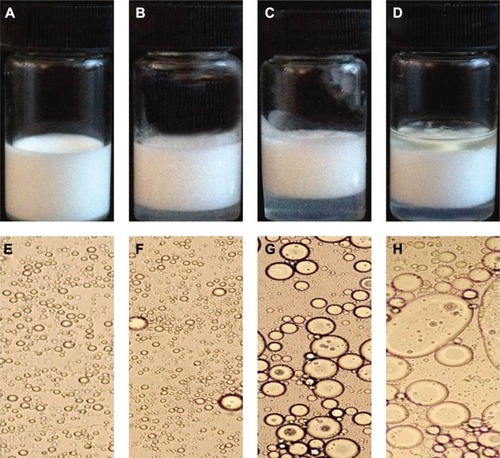
Figure 3 Appearances of TG-5FU-ME.
Abbreviations: RT, room temperature; TG-5FU-ME, thermosensitive gel-mediated 5FU water-in-oil microemulsion.
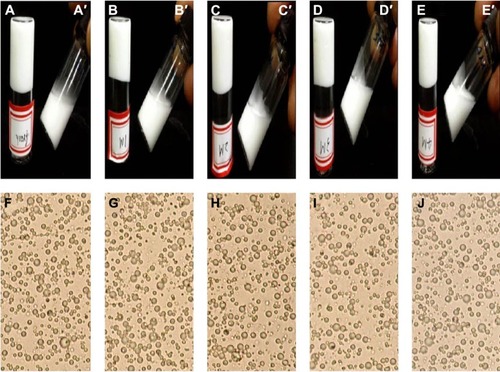
Figure 4 Release profiles of 5FU formulations in vitro.
Abbreviations: 5FU, 5-fluorouracil; TG-5FU-ME, thermosensitive gel-mediated 5FU water-in-oil microemulsion.
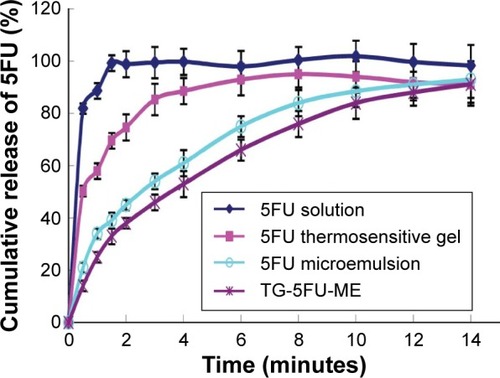
Figure 5 AP to BL (A→B) and BL to AP (B→A) transport of 5FU.
Abbreviations: AP, apical; BL, basolateral; 5FU, 5-fluorouracil; SD, standard deviation.
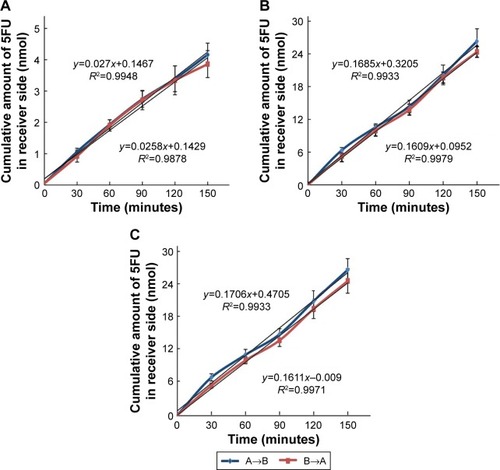
Figure 6 Formulation change affects transportation efficacy of 5FU crossing the Caco-2 cell monolayers.
Abbreviations: 5FU, 5-fluorouracil; HBSS, Hank’s buffered salt solution; HPLC, high-performance liquid chromatography; TG-5FU-ME, thermosensitive gel-mediated 5FU water-in-oil microemulsion; SD, standard deviation.
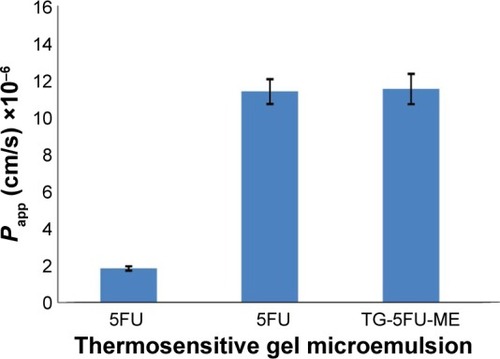
Figure 7 Absorption of 5FU in vitro.
Abbreviations: AP, apical; BL, basolateral; 5FU, 5-fluorouracil; TG-5FU-ME, thermosensitive gel-mediated 5FU water-in-oil microemulsion; SD, standard deviation.
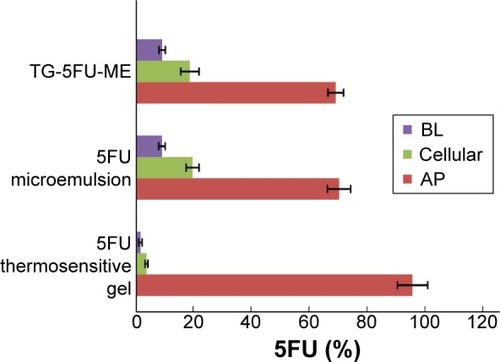
Figure 8 Rectal retention time.
Abbreviations: 5FU, 5-fluorouracil; TG-5FU-ME, thermosensitive gel-mediated 5FU water-in-oil microemulsion; cpx, count per second.
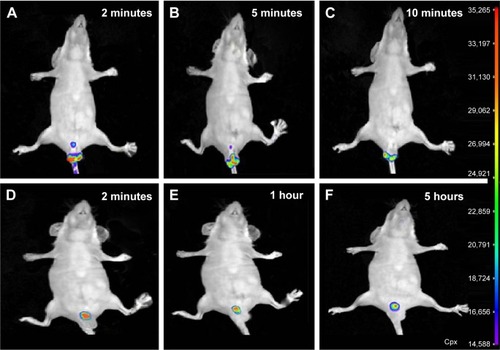
Figure 9 Tissue distribution of 5FU.
Abbreviations: 5FU, 5-fluorouracil; TG-5FU-ME, thermosensitive gel-mediated 5FU water-in-oil microemulsion; SD, standard deviation.
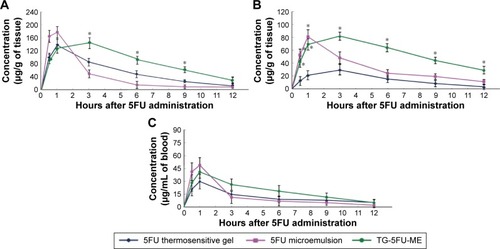
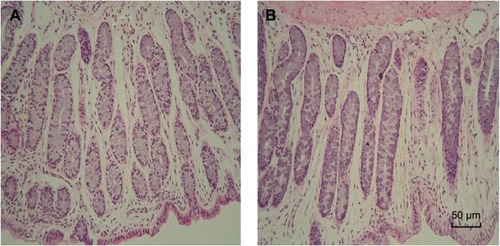
Figure 10 The morphology of rectal tissues after exposure to 5FU thermosensitive gel and TG-5FU-ME.
Abbreviations: 5FU, 5-fluorouracil; H&E, hematoxylin and eosin; TG-5FU-ME, thermosensitive gel-mediated 5FU water-in-oil microemulsion.