Figures & data
Figure 1 Study design (JSWOG-C2).
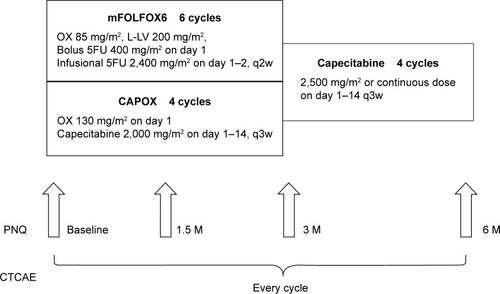
Table 1 Patient characteristics (N=86)
Figure 3 CONSORT diagram for this study.
Abbreviation: CONSORT, consolidated standards of reporting trials.
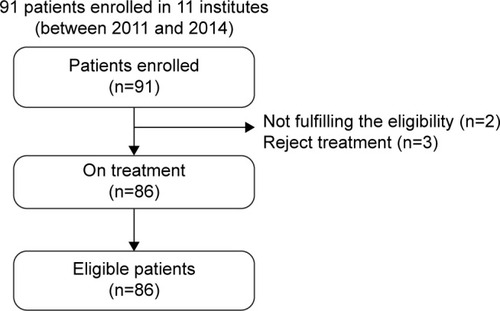
Table 2 Proportion of patients completing treatment and dose of oxaliplatin in the treatments
Table 3 Adverse events
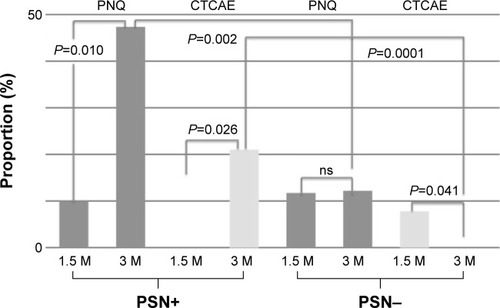
Figure 4 Frequency of neurotoxicity at four points: baseline and months 1.5, 3, and 6 after induction of adjuvant chemotherapy.
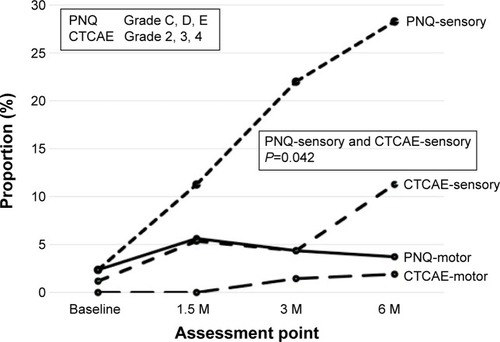
Table 4 Correlation between PNQ and CTCAE by Spearman’s correlation coefficient
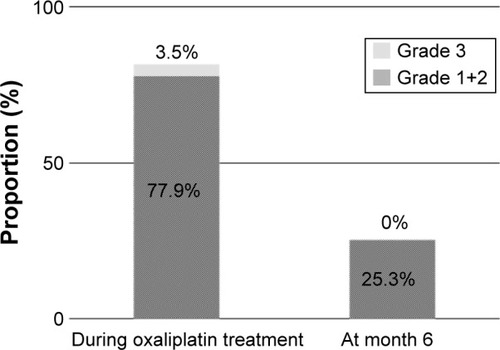
Figure 5 PSN during oxaliplatin treatment and at month 6.