Figures & data
Figure 1 Structures of considered AEDs and of newly designed final compounds 1–6.
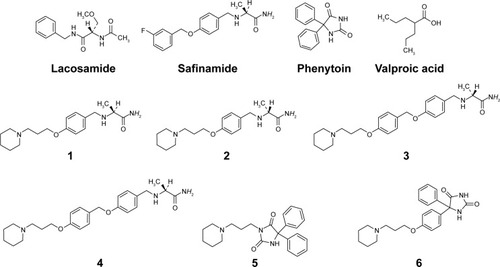
Table 1 In vitro affinities at hH1Rs, hH2Rs, and hH4Rs and in vivo anticonvulsant effects of final compounds 1–6
Figure 2 Protective effect of acute systemic injection of H3R ligands 1–6 on MES-induced convulsions in rats.
Abbreviations: PHT, phenytoin; PIT, pitolisant; THLE, tonic hind limb extension; MES, maximal electroshock; IP, intraperitoneally; SEM, standard error of the mean; s, seconds.
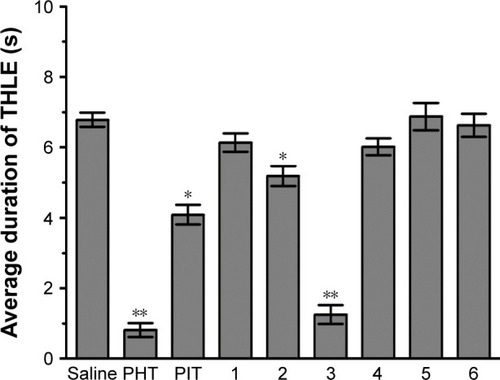
Figure 3 Dose-dependent protective effect of H3R ligand 3 against MES-induced convulsions.
Abbreviations: THLE, tonic hind limb extension; MES, maximal electroshock; SEM, standard error of the mean; s, seconds.
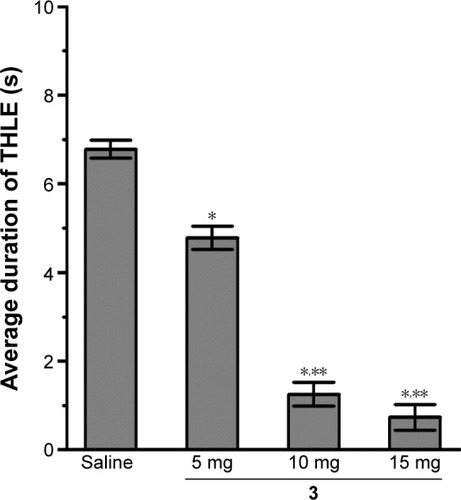
Figure 4 Effect of RAMH (10 mg/kg, IP) pretreatment on the protection by H3R ligand 3 (10 mg/kg, IP) on MES-induced convulsions.
Abbreviations: THLE, tonic hind limb extension; MES, maximal electroshock; RAMH, R-(α)-methyl-histamine; IP, intraperitoneally; SEM, standard error of the mean; s, seconds.
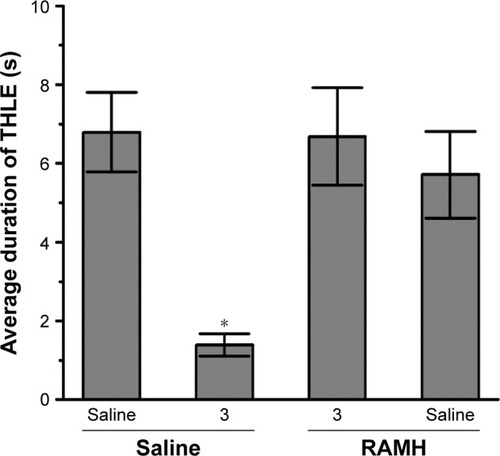
Figure 5 Protective effect of H3R ligands 1–6 pretreatment on PTZ-induced convulsions in rats.
Abbreviations: VPA, valproic acid; PIT, pitolisant; PTZ, pentylenetetrazole; IP, intraperitoneally; SEM, standard error of the mean.
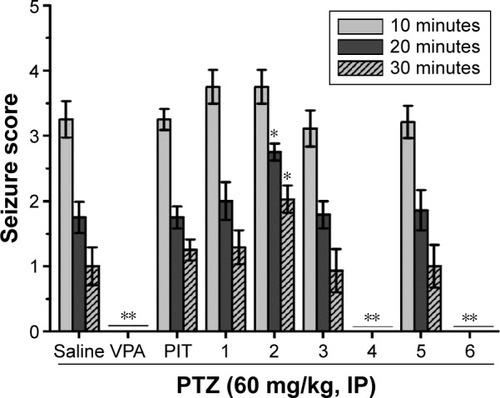
Figure 6 Dose-dependent protective effect of H3R ligands 2, 4, and 5 against PTZ-induced convulsions.
Abbreviations: PTZ, pentylenetetrazole; SEM, standard error of the mean.
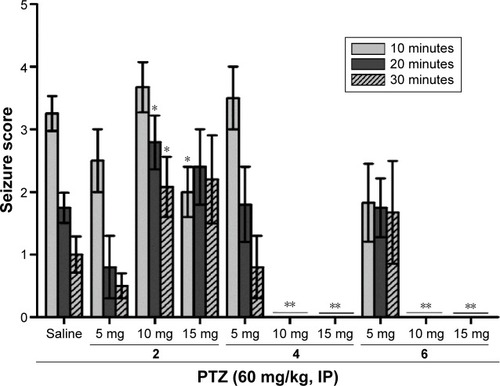
Figure 7 Effect of RAMH (10 mg/kg, IP) pretreatment on the protection by H3R ligand 4 (10 mg/kg, IP) on PTZ-induced convulsions in rats.
Abbreviations: PTZ, pentylenetetrazole; RAMH, R-(α)-methyl-histamine; IP, intraperi-toneally.
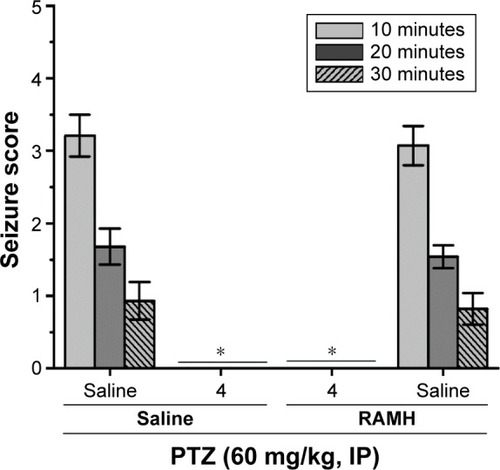
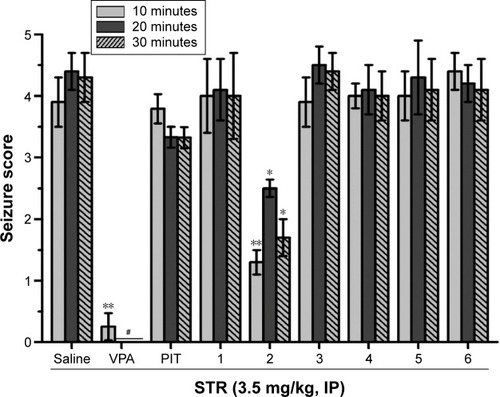
Figure 8 Protective effect of H3R ligands 1–6 pretreatment on STR-induced convulsions in rats.
Abbreviations: STR, strychnine; VPA, valproic acid; PIT, pitolisant; IP, intraperitoneally; SEM, standard error of the mean.
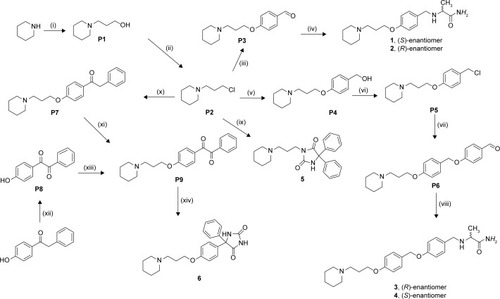
Scheme 1 Synthesis of precursors P1–P9 and final H3R ligands 1–6.
Abbreviation: RT, room temperature.