Figures & data
Table 1 Demographic characteristics at screening – MTD and TQT studies
Table 2 Plasma pharmacokinetics of lesinurad following a single dose of lesinurad 800 mg, 1,200 mg, or 1,600 mg to healthy male and female subjects – MTD and TQT study
Figure 1 Dose linearity assessment of Cmax (A) and AUC[0–inf] (B) for lesinurad in males and females – MTD study.
Abbreviations: AUC[0–inf], area under the plasma concentration–time curve from zero to infinity; Cmax, maximum observed plasma concentration; MTD, maximum tolerated dose; SE, standard error.
![Figure 1 Dose linearity assessment of Cmax (A) and AUC[0–inf] (B) for lesinurad in males and females – MTD study.](/cms/asset/7c354f9e-e417-4a64-835a-1506ae71c60f/dddt_a_12173064_f0001_b.jpg)
Figure 2 Placebo-corrected change from baseline QTcI versus sample time.
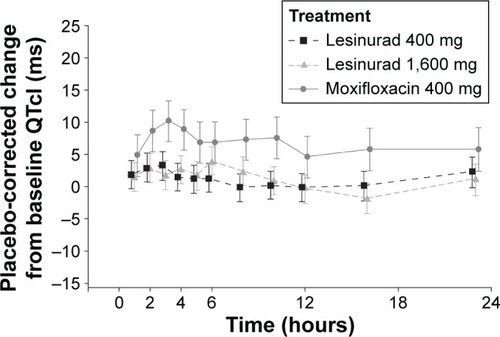
Figure 3 QTcI placebo-corrected change from baseline versus lesinurad plasma concentration.
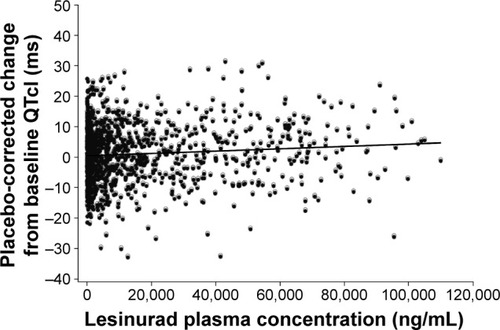
Table 3 Effect of sex on lesinurad pharmacokinetics following lesinurad dosing at 400 mg and 1,600 mg – TQT study
