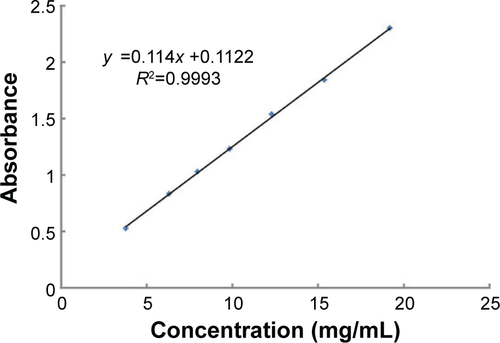
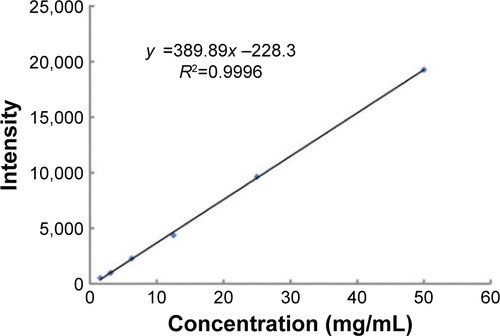
Figures & data
Table 1 Some interesting clinical combinations and the outcomes of CDDP-based therapy
Figure 1 Preparation of MSNS-6MP/CDDP.
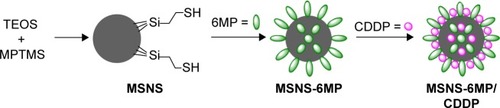
Figure 2 DSC spectra of MSNS, CDDP, 6MP, MSNS + CDDP, MSNS + 6MP, MSNS + CDDP + 6MP and MSNS-6MP/CDDP.
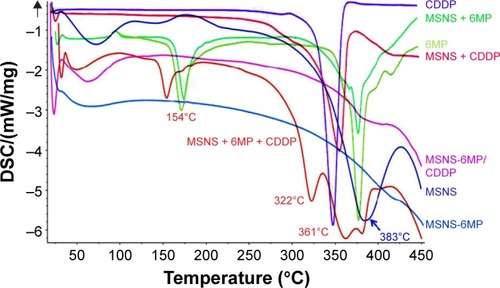
Figure 3 TEM images of (A) MSNS, (B) MSNS-6MP and (C) MSNS-6MP/CDDP.
Abbreviations: TEM, transmission electron microscope; MSNS, SH surfaced mesoporous silica nanoparticles; 6MP, 6-mercaptopurine; CDDP, cisplatin; MSNS-6MP, 6MP covalently modified MSNS; MSNS-6MP/CDDP, CDDP-loaded MSNS-6MP.
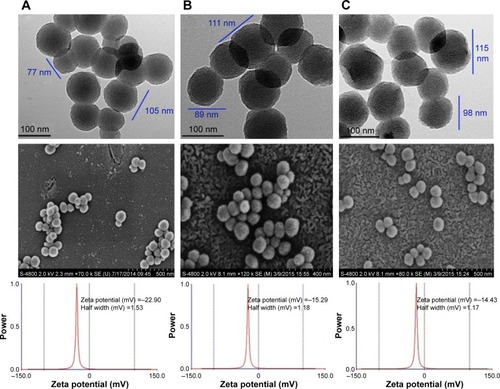
Figure 4 Accumulative release of 6MP from MSNS-6MP.
Abbreviations: 6MP, 6-mercaptopurine; MSNS, SH surfaced mesoporous silica nanoparticles; GSH, glutathione; PBS, phosphate buffered saline; DTT, dithiolthreitol; MSNS-6MP, 6MP surfaced MSNS.
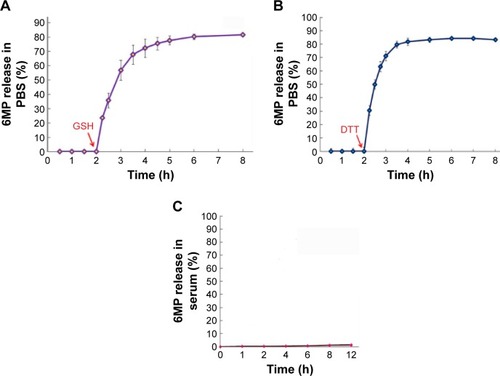
Figure 5 The release profile of CDDP from MSNS-6MP/CDDP.
Abbreviations: CDDP, cisplatin; MSNS, SH surfaced mesoporous silica nanoparticles; 6MP, 6-mercaptopurine; MSNS-6MP, 6MP covalently modified MSNS; MSNS-6MP/CDDP, CDDP-loaded MSNS-6MP; PBS, phosphate buffered saline.
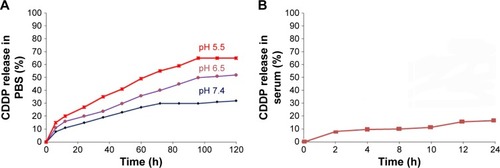
Figure 6 Survival numbers of S180 mice and healthy ICR mice.
Abbreviations: CMCNa, sodium carboxyl methyl cellulose; CDDP, cisplatin; 6MP, 6-mercaptopurine; MSNS, SH surfaced mesoporous silica nanoparticles; MSNS-6MP, 6MP covalently modified MSNS; MSNS-6MP/CDDP, CDDP-loaded MSNS-6MP.
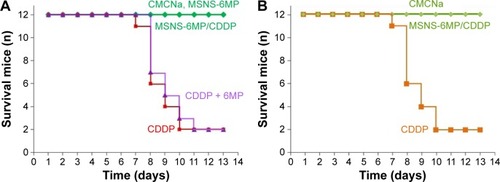
Figure 7 Tumor volumes of the treated mice.
Abbreviations: CMCNa, sodium carboxyl methyl cellulose; CDDP, cisplatin; 6MP, 6-mercaptopurine; MSNS, SH surfaced mesoporous silica nanoparticles; MSNS-6MP, 6MP covalently modified MSNS; MSNS-6MP/CDDP, CDDP-loaded MSNS-6MP.
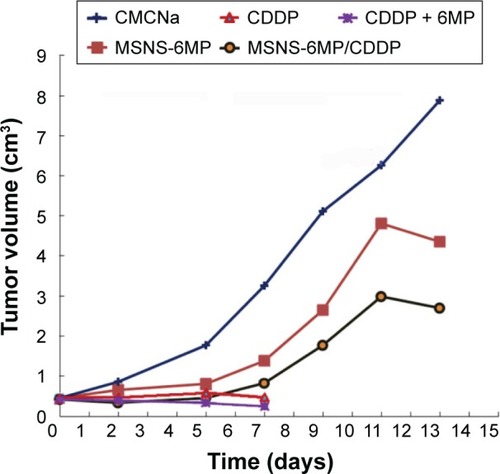
Figure 8 Tumor weights of S180 mice treated with CMCNa, MSNS-6MP and MSNS-6MP/CDDP, n=12.
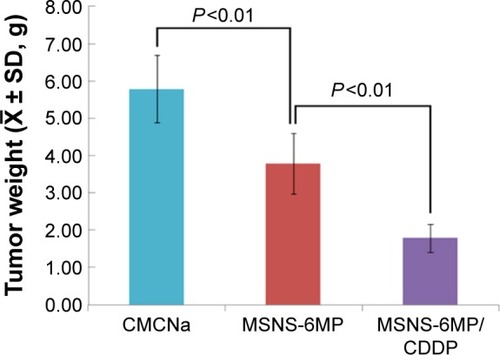
Table 2 Body weight (mean ± SD, g) and ratio of organ weight/body weight (mean ± SD, %) of S180 mice treated by 6MP plus CDDP and MSNS-6MP/CDDP
Figure 9 Serum ALT, AST and Cr levels of the mice treated with CMCNa, CDDP, CDDP plus 6MP and MSNS-6MP/CDDP.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; Cr, creatinine; CMCNa, sodium carboxyl methyl cellulose; CDDP, cisplatin; 6MP, 6-mercaptopurine; MSNS, SH surfaced mesoporous silica nanoparticles; MSNS-6MP, 6MP covalently modified MSNS; MSNS-6MP/CDDP, CDDP-loaded MSNS-6MP.
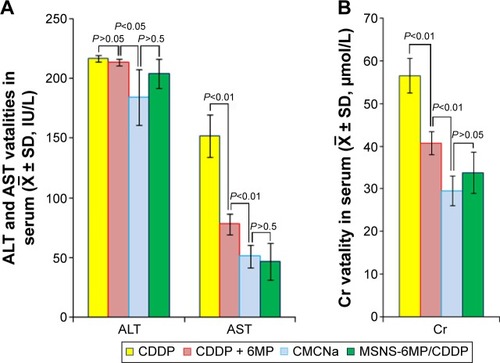
Figure 10 Effect of CMCNa, MSNS, 6MP plus CDDP and MSNS-6MP/CDDP on the left kidney histology.
Abbreviations: CMCNa, sodium carboxyl methyl cellulose; MSNS, SH surfaced mesoporous silica nanoparticles; 6MP, 6-mercaptopurine; CDDP, cisplatin; MSNS-6MP, 6MP covalently modified MSNS; MSNS-6MP/CDDP, CDDP-loaded MSNS-6MP; H&E, hematoxylin and eosin.
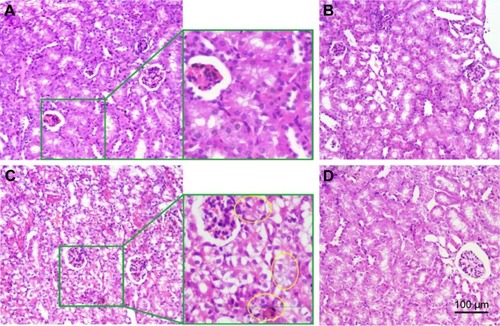
Figure 11 Effect of CMCNa, MSNS, 6MP plus CDDP and MSNS-6MP/CDDP on mouse myocardium histology.
Abbreviations: CMCNa, sodium carboxyl methyl cellulose; MSNS, SH surfaced mesoporous silica nanoparticles; 6MP, 6-mercaptopurine; CDDP, cisplatin; MSNS-6MP, 6MP covalently modified MSNS; MSNS-6MP/CDDP, CDDP-loaded MSNS-6MP; H&E, hematoxylin and eosin.
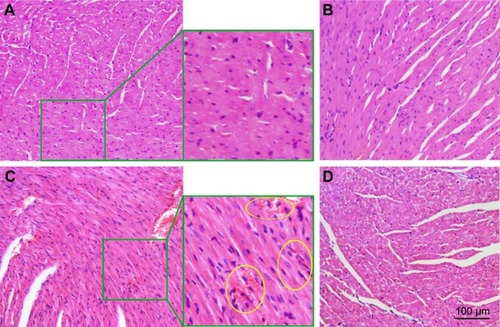
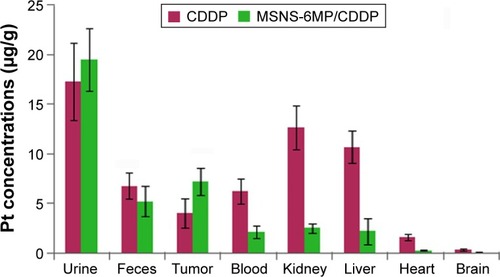
Figure 12 Distribution of Pt in S180 mice treated by MSNS-6MP/CDDP and CDDP (μg/g).
Abbreviations: MSNS, SH surfaced mesoporous silica nanoparticles; 6MP, 6-mercaptopurine; CDDP, cisplatin.