Figures & data
Figure 1 Study design.
Abbreviation: CKD-828, a fixed-dose combination of S-amlodipine and telmisartan.
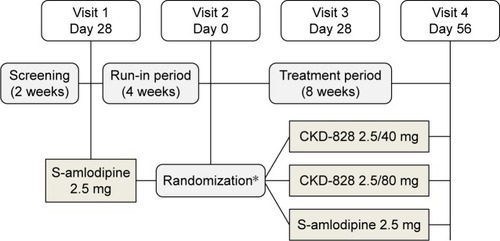
Figure 2 Summary of patient disposition.
Abbreviations: CKD-828, a fixed-dose combination of S-amlodipine and telmisartan; FA, full analysis; PP, per-protocol.
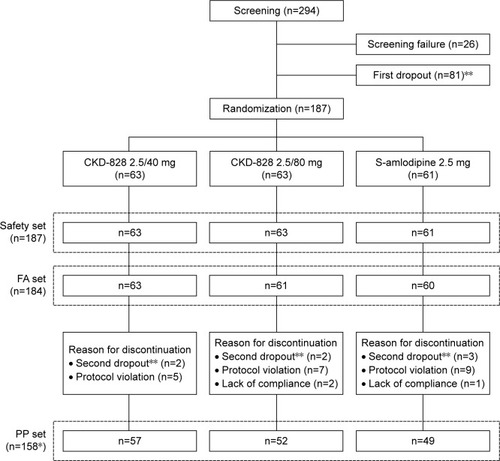
Table 1 Baseline characteristics
Table 2 Change in sitting diastolic blood pressure from baseline in each treatment group at Weeks 4 and 8
Figure 3 Effect of 8 weeks of treatment with CKD-828 2.5/40 mg and CKD-828 2.5/80 mg compared with S-amlodipine 2.5 mg on the change from baseline in sitDBP or sitSBP.
Abbreviations: BP, blood pressure; CKD-828, a fixed-dose combination of S-amlodipine and telmisartan; sitDBP, sitting diastolic blood pressure; sitSBP, sitting systolic blood pressure.
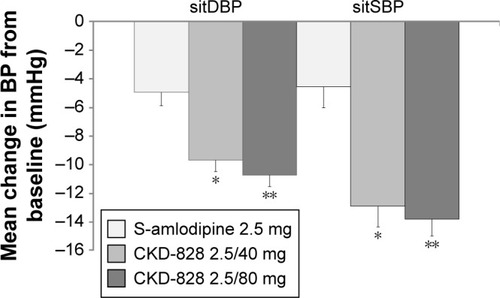
Table 3 Control rate and response rate by group
Figure 4 (A) Control and (B) Response rate of CKD-828 2.5/40 mg and CKD-828 2.5/80 mg compared with S-amlodipine 2.5 mg at Weeks 4 and 8 from baseline. *P<0.01 versus S-amlodipine monotherapy. **P<0.001 versus S-amlodipine monotherapy.
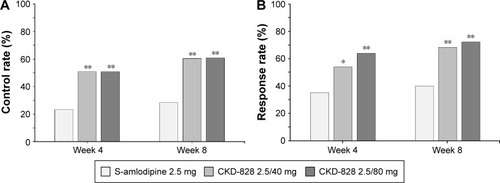
Table 4 Summary of adverse events during the study period according to treatment group
