Figures & data
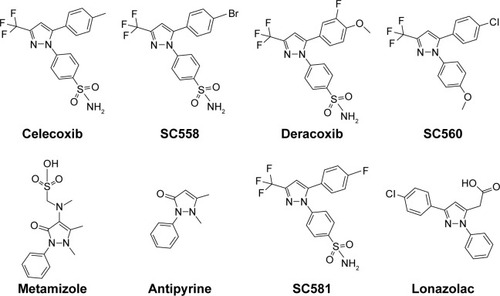
Figure 2 Strategy for design of target compound with structural resemblance of reference ligand.
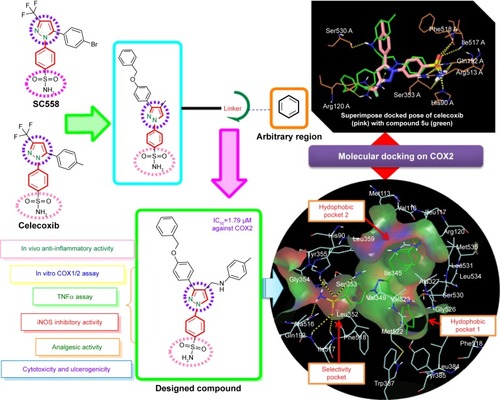
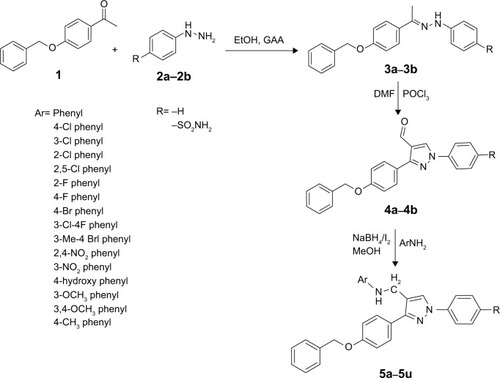
Table 1 In vivo results of N-((3-(4-(benzyloxy)phenyl)-1-phenyl-1H-pyrazol-4-yl)methyl) aniline derivatives (5a–5u)
Figure 3 Histopathological study of stomachs of rats treated with standard drug (ibuprofen) and active compounds in comparison to healthy controls.
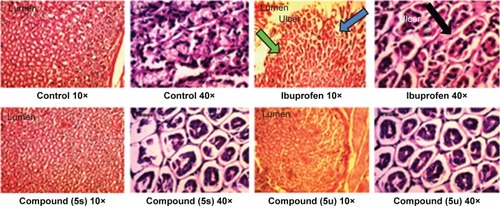
Table 2 Results of in vitro COX1/COX2 enzyme inhibition, NO-inhibition assay, and cytotoxicity study
Figure 4 In vitro TNFα assay of synthesized compound.
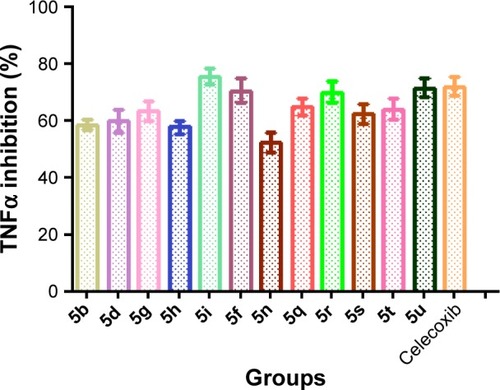
Table 3 In silico docking score of N-((3-(4-(benzyloxy)phenyl)-1-phenyl-1H-pyrazol-4-yl)methyl) aniline derivatives (5a–5u)
Figure 5 2-D LigPlot interaction diagrams.
Abbreviation: 2-D, two-dimensional.
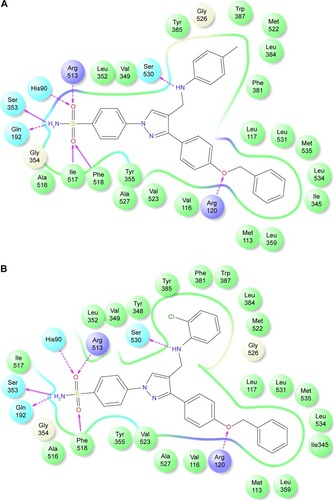
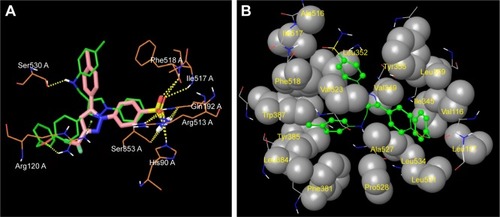
Figure 6 Docked pose of 5s and 5u.
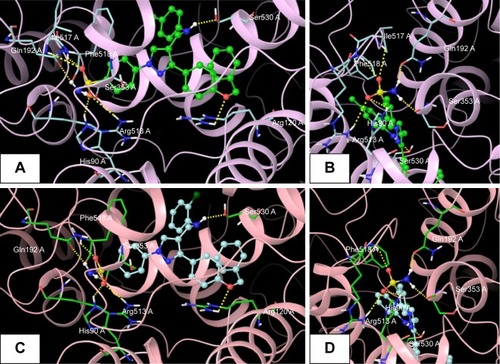
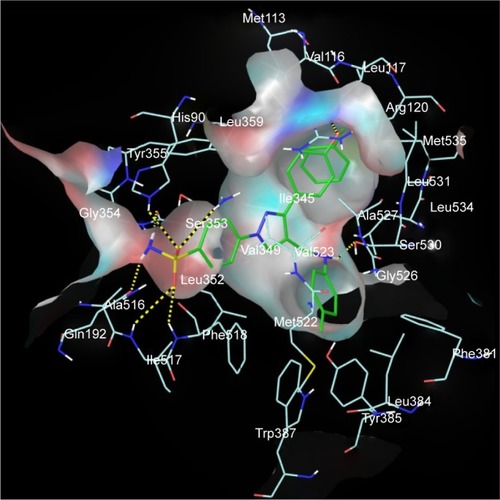
Figure 7 Superimposed and hydrophobic interactions of 5u.