Figures & data
Table 1 Formulation of CRS by L9 (34) statistic experiment
Table 2 Orthogonal analysis of L9 (34) statistic experiment
Figure 1 The agarose gel electrophoresis of VEGF-siRNA and CRS with different concentrations.
Abbreviation: siRNA, small interfering RNA.
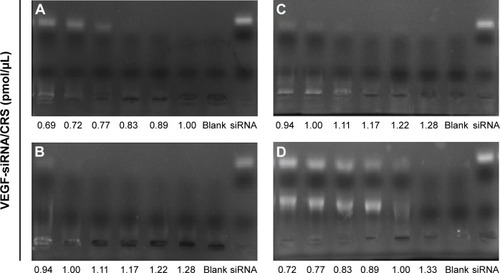
Figure 2 Faraday-Tyndall effect of control and CRS (25%, 50%, 75%, 100%).
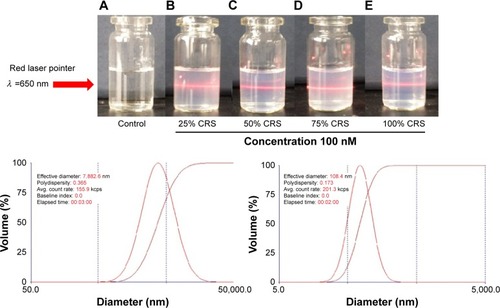
Figure 3 Nano-properties of CRS and VEGF-siRNA/CRS.
Abbreviation: siRNA, small interfering RNA.
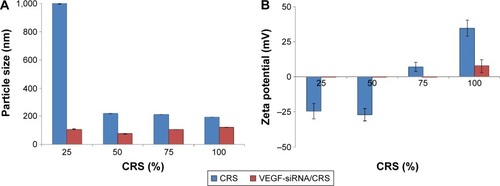
Figure 4 TEM (A–D) and SEM (E–H) images of VEGF-siRNA/CRS.
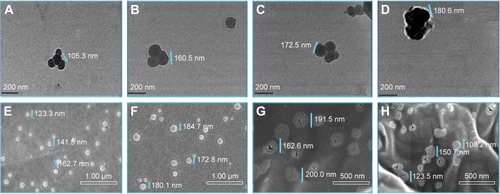
Figure 5 Confocal image (×104~105) of in vitro cellular uptakes of VEGF-siRNA/CRS at 2 h and 4 h.
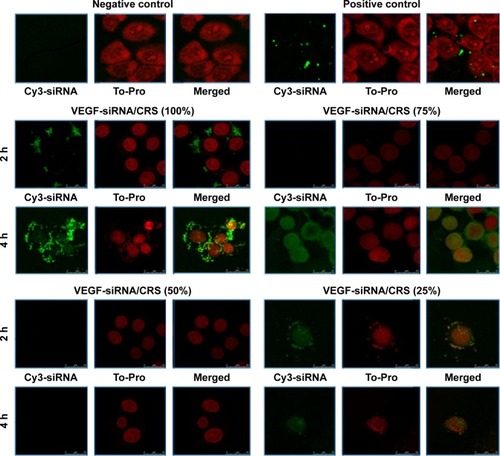
Figure 6 Release of VEGF-siRNA and CRS from VEGF-siRNA/CRS. Note: Data are presented as mean ± SD (n=3).
Figure 7 In vitro inhibition of HeLa cells treated by VEGF-siRNA/CRS (n=3).
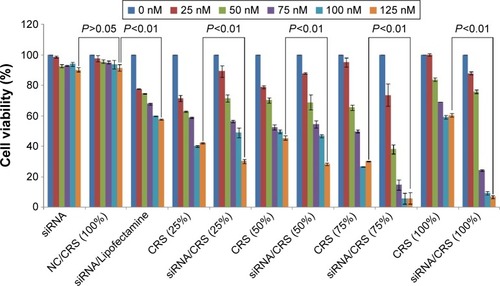
Figure 8 ELISA results for VEGF protein expression of HeLa cells treated with VEGF-siRNA/CRS.
Abbreviations: NC, negative control; siRNA, small interfering RNA; ELISA, enzyme-linked immunosorbent assay; SD, standard deviation.
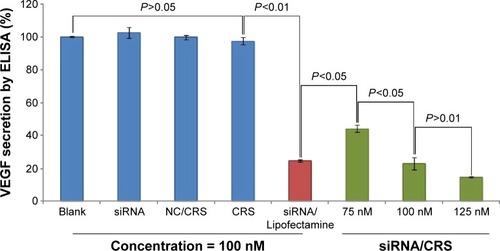
Figure 9 Gene-silencing efficiency of VEGF-siRNA/CRS on HeLa cells.
Abbreviations: NC, negative control; siRNA, small interfering RNA; SD, standard deviation.
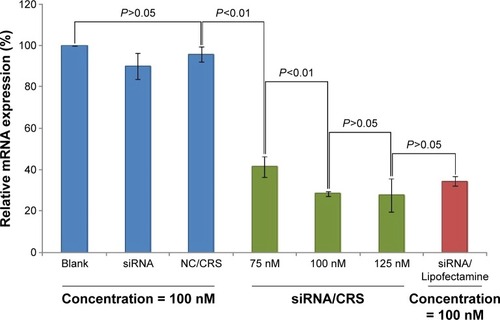
Figure 10 The coronal sections of the tumor from the right armpit of mice.
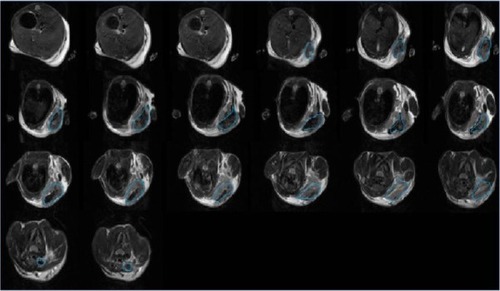
Figure 11 Imaging of mice from animal fluorescence system.
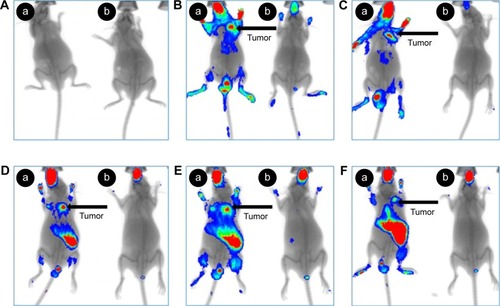
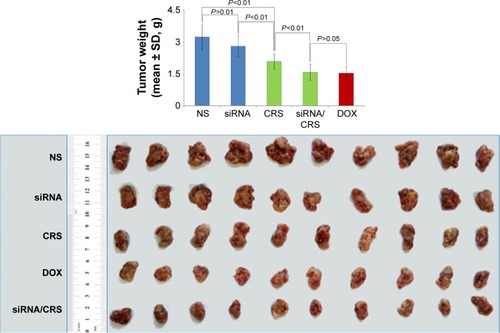
Figure 12 In vivo antitumor effect of VEGF-siRNA/CRS.
Abbreviations: DOX, doxorubicin; NS, normal saline; SD, standard deviation; siRNA, small interfering RNA.
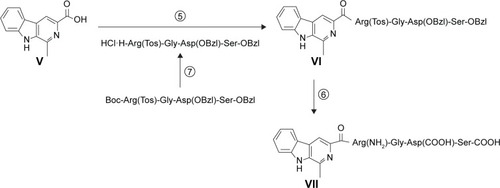
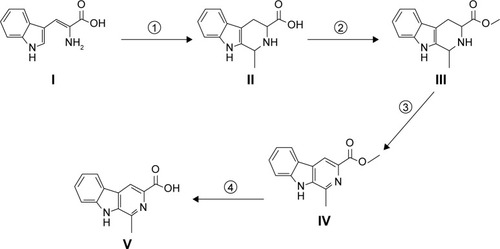
Scheme 1 Synthesis of 1-methyl-β-caboline-3-carboxylic acid.
Scheme 3 Synthesis of 1-methyl-β-caboline-3-RGDS with carboxyl and amino terminal.
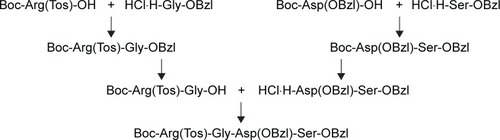
Abbreviations: DCC, dicyclohexylcarbodiimide; HOBt, 1-hydroxybenzotriazole; NMM, N-methylmorpholine; TFA, trifluoroacetic acid; TfOH, trifluoromethanesulfonic acid; EtOAc, ethyl acetate.