Figures & data
Figure 1 Molecular structures of synthesized sterols.
Abbreviations: Triol, Androst-3β,5α,6β-triol; YC-1, Cholest-24-ene-3β,5α,6β,19-tetrol; YC-10, Androst-2β,3α,5α-trihydroxy-6-one; YC-5, Cholest-3β,5α,6β-triol
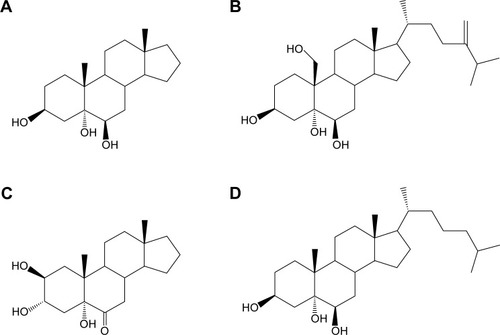
Table 1 Solubility of Triol in different BS/PC-MM systems with different weight ratios of BS to PC
Table 2 Solubility of Triol in different BS/PC-MM systems with different weight ratios of BS to PC at a fixed total concentration
Figure 2 Solubility of Triol in different BS/PC-MM systems with their optimized ratios and varied total concentrations of BS and PC (n=3).
Abbreviations: BS, bile salt; BS/PC-MM, bile salt/phosphatidylcholine mixed micelles; EPC, egg phosphatidylcholine; MM, mixed micelles; PC, phosphatidylcholine; SGC, sodium glycocholate; SPC, soya phosphatidylcholine; SBA-Na, swine bile acid-sodium salt; SDC, sodium deoxycholate; Triol, Androst-3β,5α,6β-triol.
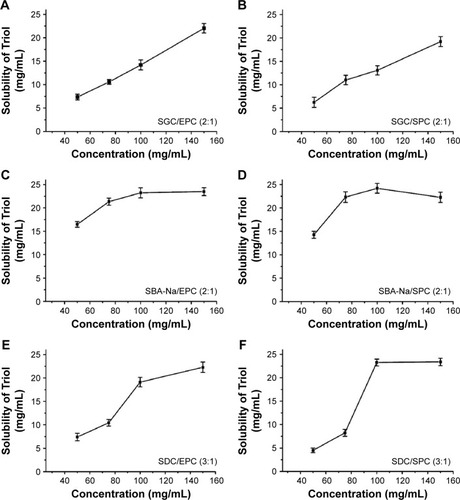
Table 3 Characterization of different Triol-loaded BS/PC-MM systems at their optimized ratios and total concentrations
Table 4 Stability of varied Triol-loaded BS/PC-MM systems at their optimized ratios and total concentrations at 25°C±2°C
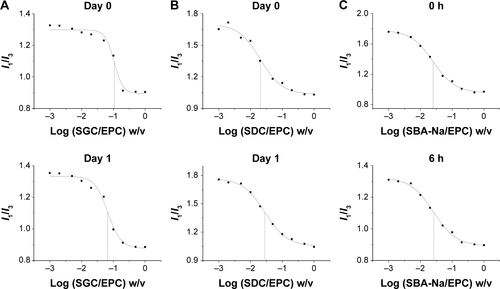
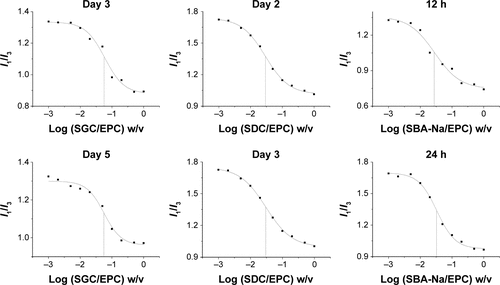
Figure 3 The change of CMC of Triol-loaded BS/PC-MM systems under accelerated testing conditions.
Abbreviations: CMC, critical micelle concentration; BS/PC-MM, bile salt/phosphatidylcholine mixed micelles; EPC, egg phosphatidylcholine; RH, relative humidity; SBA-Na, swine bile acid-sodium salt; SDC, sodium deoxycholate; SGC, sodium glycocholate; SPC, soya phosphatidylcholine; Triol, Androst-3β,5α,6β-triol.
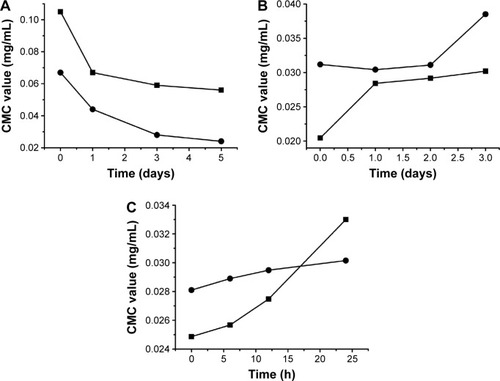
Figure 4 Solubility of synthesized sterols in the optimized SGC/EPC-MM system with ratio in weight 2:1 and the total concentration of 100 mg/mL (n=3).
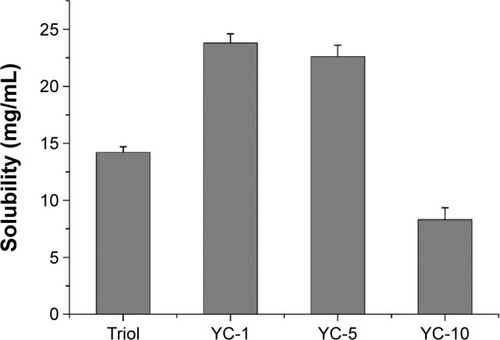
Figure S1 Plot of the fluorescence of pyrene I1/I3 intensity ratio versus concentration of surfactant. The CMC corresponds to the center of the sigmoid.
Abbreviations: CMC, critical micelle concentration; I, fluorescence intensity of pyrene; I1/I3, intensity of the third to the first peak of pyrene.
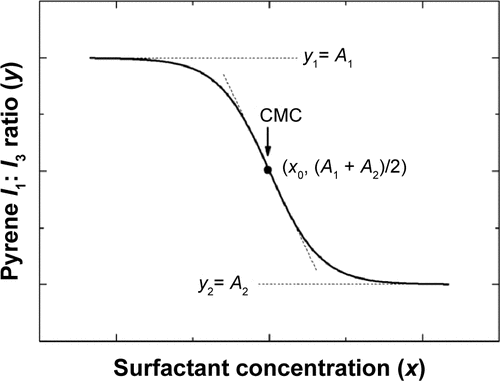
Figure S2 Representative plots of the fluorescence of pyrene I1/I3 intensity ratio versus concentration of BS/PC-MM systems under accelerated conditions.
Abbreviations: BS/PC-MM, bile salt/phosphatidylcholine mixed micelles; CMC, critical micelles concentration; EPC, egg phosphatidylcholine; I, fluorescence intensity of pyrene; I1/I3, intensity of the third to the first peak of pyrene; MM, mixed micelles; SBA-Na, swine bile acid-sodium salt; SDC, sodium deoxycholate; SGC, sodium glycocholate; w/v, weight/volume.