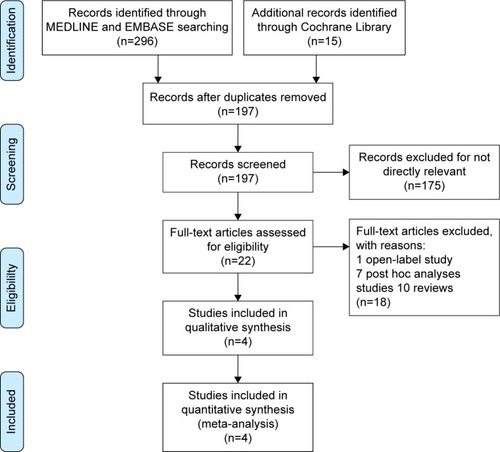
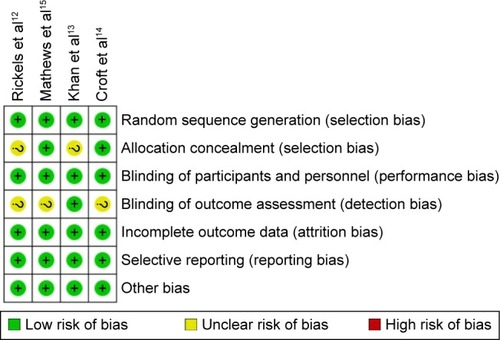
Figures & data
Table 1 Characteristics of the included studies and outcome events
Figure 2 MADRS change from baseline.
Notes: **P<0.01, ***P<0.001.
Abbreviations: RR, relative risk; CI, confidence interval; MADRS, Montgomery–Asberg Depression Rating Scale.
Abbreviations: RR, relative risk; CI, confidence interval; MADRS, Montgomery–Asberg Depression Rating Scale.
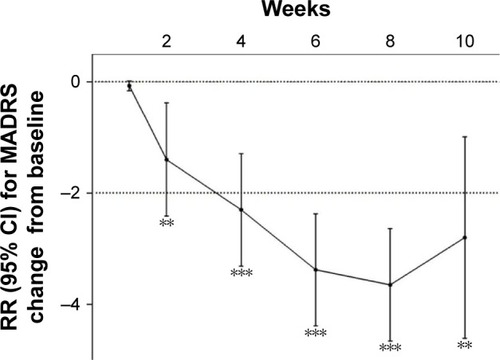
Figure 3 Secondary efficacy outcomes.
Notes: (A) Montgomery–Asberg Depression Rating Scale (MADRS), (B) Hamilton Rating Scale for Anxiety (HAM-A), (C) Clinical Global Impressions-Improvement of Illness (CGI-I), and (D) Clinical Global Impressions-Severity of Illness (CGI-S).
Abbreviations: CI, confidence interval; SD, standard deviation; df, degrees of freedom.
Abbreviations: CI, confidence interval; SD, standard deviation; df, degrees of freedom.
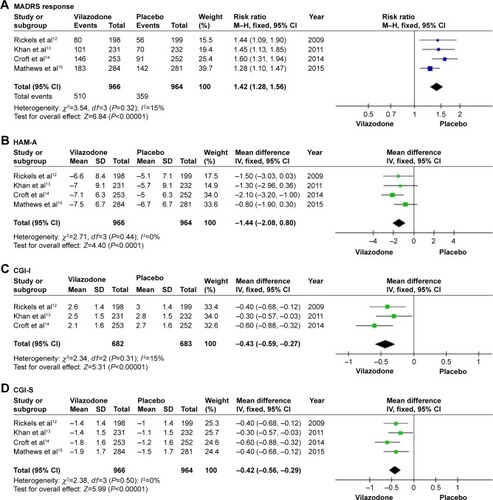
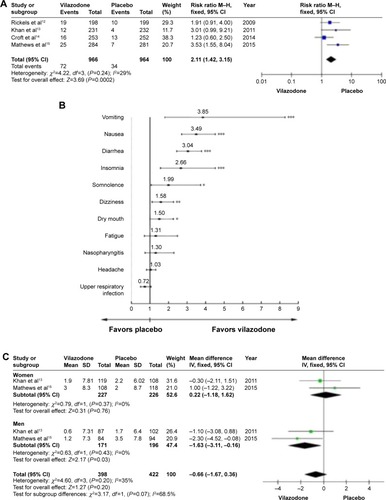
Figure 4 Adverse effects.
Notes: (A) Discontinued rate due to adverse events, (B) frequently reported adverse events, and (C) sexual dysfunction. *P<0.05; **P<0.01; ***P<0.001.
Abbreviations: CI, confidence interval; SD, standard deviation; df, degrees of freedom.
Abbreviations: CI, confidence interval; SD, standard deviation; df, degrees of freedom.