Figures & data
Figure 1 Stepwise illustration of DL-CDDS as a platform for colon targeting.
Abbreviations: DL-CDDS, double layer-coated colon-specific drug delivery system; CTN, chitosan; CTNase, chitosanase.
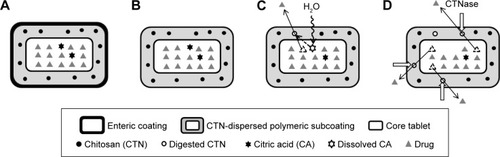
Table 1 Composition of various DL-CDDS formulations
Table 2 Variables used in the 2-LFD method
Table 3 Physical properties of core tablets
Figure 2 Dissolution characteristics of various formulations.
Abbreviations: CA, citric acid; CTN, chitosan; CTNase, chitosanase; EE, Eudragit E100; LXP, loxoprofen sodium; SCF, simulated colonic fluid; SGF, simulated gastric fluid; SIF, simulated intestinal fluid; CA0, citric acid-free tablet; CA15, CA 15 mg; CA30, CA 30 mg.
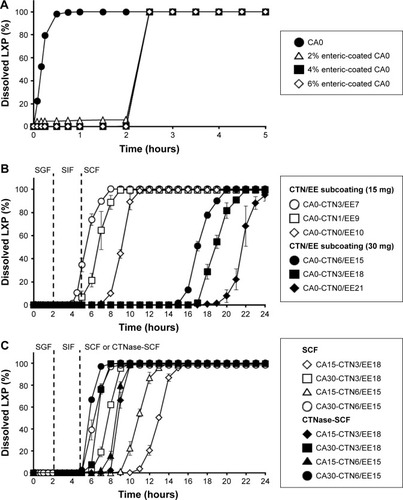
Figure 3 Comparison for dissolution efficiency of various formulations.
Abbreviations: CA, citric acid; CTN, chitosan; CTNase, chitosanase; DE, dissolution efficiency; DEcolon, DE in the colon; EE, Eudragit E100; SCF, simulated colonic fluid; CA0, citric acid-free tablet; CA15, CA 15 mg; CA30, CA 30 mg.
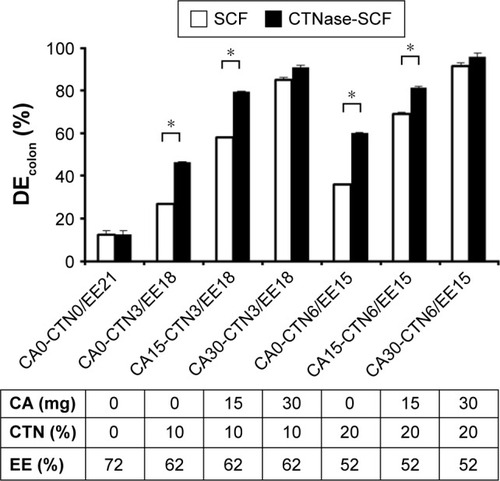
Table 4 Experimental design and responses observed from randomized runs in the 2-LFD method
Table 5 Estimated effects and coefficients
Figure 4 Normal probability plots of the standardized residual (A) and Pareto charts of the standardized effect (B).
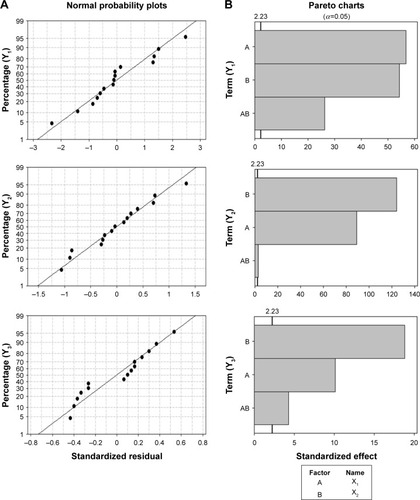
Figure 5 Main effect plot (A) and interaction plot (B).
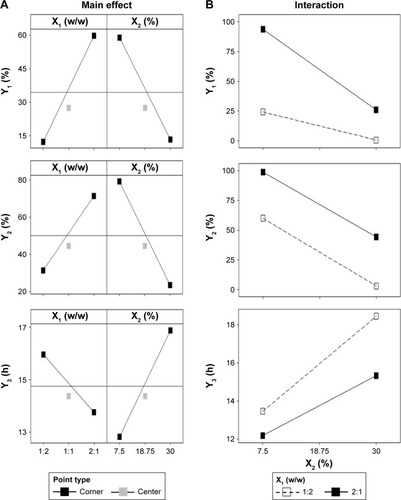
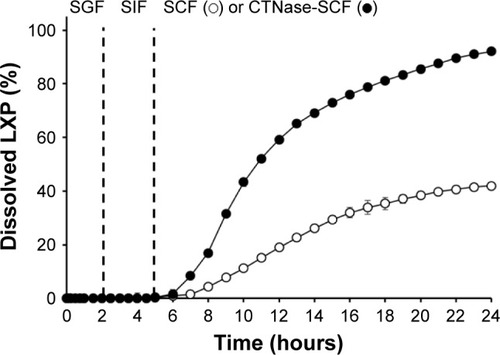
Figure 6 Dissolution profile of the optimized DL-CDDS.
Abbreviations: CTNase, chitosanase; DL-CDDS, double layer-coated colon-specific drug delivery system; LXP, loxoprofen sodium; SCF, simulated colonic fluid; SGF, simulated gastric fluid; SIF, simulated intestinal fluid.