Figures & data
Table 1 Physicochemical characterization of MHY-SLNs
Figure 1 In vitro characterization of MHY-SLNs.
Abbreviations: MHY-SLNs, MHY498-loaded solid lipid nanoparticles; endo, endothermic.
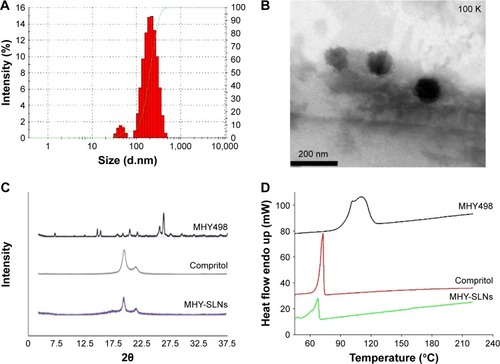
Figure 2 In vitro release profile of MHY498-loaded solid lipid nanoparticles. Notes: A drug-release study was performed using the dialysis bag-diffusion technique. Results are presented as the mean ± SD (n=3).
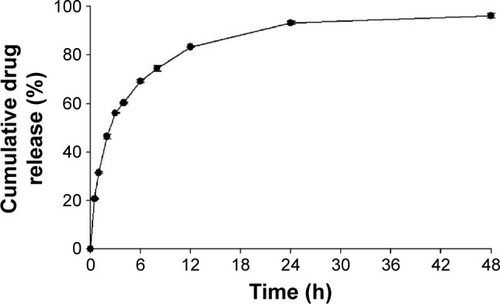
Table 2 Flux determination of MHY-SLNs and MHY solution at different time intervals
Figure 3 In vitro skin permeation of MHY solution and MHY-SLNs.
Abbreviations: MHY-SLNs, MHY498-loaded solid lipid nanoparticles; SD, standard deviation.
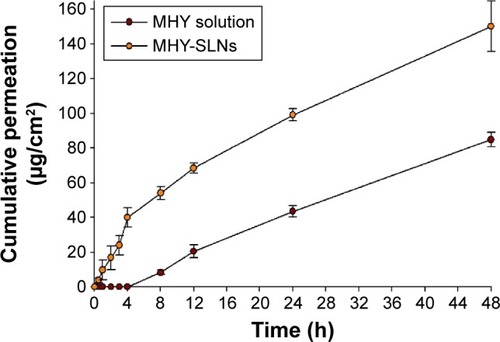
Figure 4 Proposed permeation mechanism of MHY498 from MHY-SLNs matrix through loose corneocyte packing of stratum corneum after skin application.
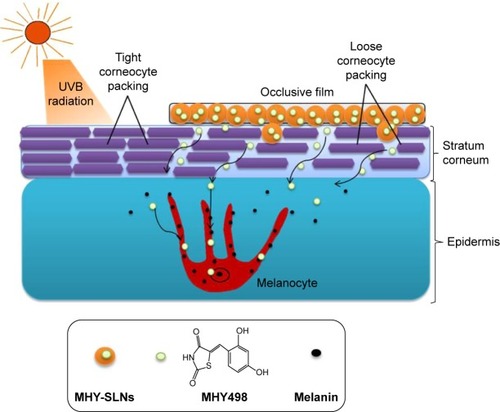
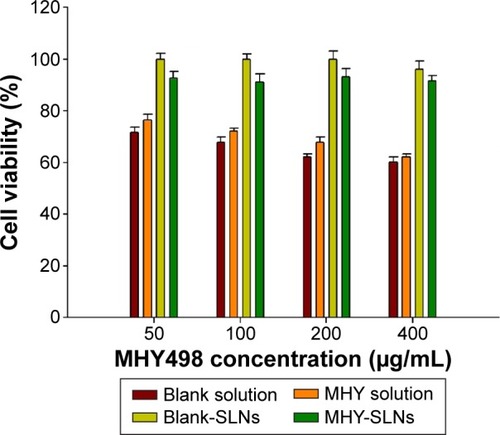
Figure 5 In vivo evaluation of MHY-SLNs.
Abbreviations: MHY-SLNs, MHY498-loaded solid lipid nanoparticles; SD, standard deviation; UV control, ultraviolet control.
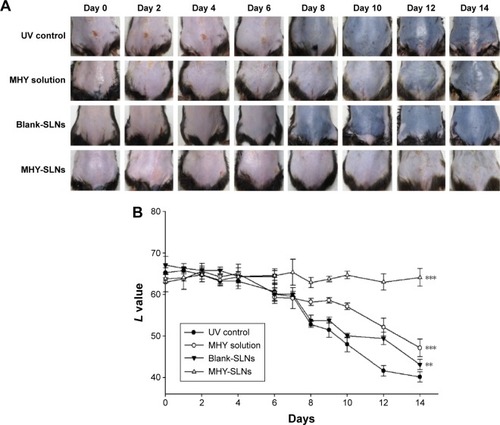
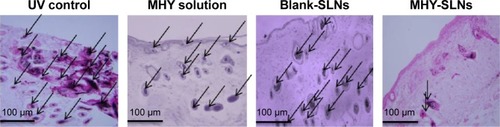
Figure 6 Fontana–Masson-stained sections of C57BL/6 skin from the UV control, MHY solution, and MHY-SLNs groups.
Abbreviations: MHY-SLNs, MHY498-loaded solid lipid nanoparticles; UV control, ultraviolet control.