Figures & data
Figure 1 Molecular structure of DFP-11207 and 5-fluorouracil.
Abbreviation: DFP-11207, 5-chloro-2-(3-(3-(ethoxymethyl)-5-fluoro-2,6-dioxo-1,2,3,6-tetrahydopyrimidine-1-carbonyl)benzoyloxy)pyridine-4-yl-2,6-bis(propionyloxy) isoni cotinate.
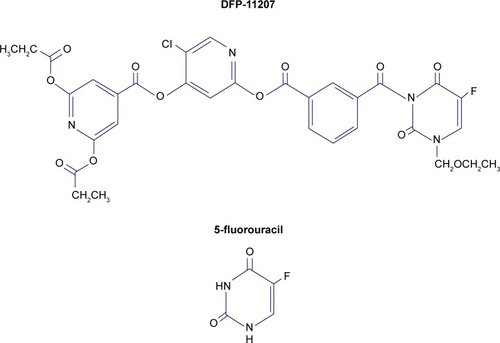
Figure 2 Enzymatic conversion of DFP-11207 to three metabolites, EM-FU, CDHP, and CTA in the serum, liver, and small intestine of rats in vitro.
Abbreviations: CDHP, 5-chloro-2,4-dihydroxypyridine; CTA, citrazinic acid; DFP-11207, 5-chloro-2-(3-(3-(ethoxymethyl)-5-fluoro-2,6-dioxo-1,2,3,6-tetrahydopyrimidine-1-carbonyl)benzoyloxy)pyridine-4-yl-2,6-bis(propionyloxy)isonicotinate; EM-FU, 1-ethoxymethyl-5-fluorouracil; HPLC, high-performance liquid chromatography.
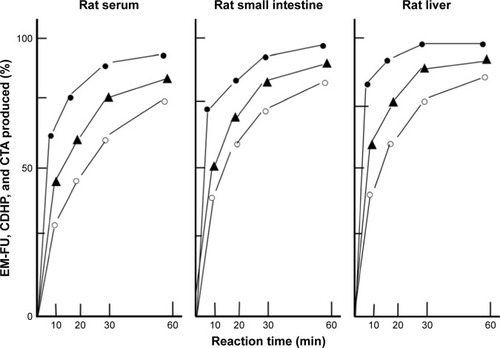
Table 1 Inhibitory effect of DFP-11207 on the activities of DPD and OPRT
Figure 3 Inhibitory effects of DFP-11207 and citrazinic acid on intracellular phosphorylation of 5-FU and its subsequent incorporation into RNA in human colorectal tumor cells.
Abbreviations: 5-FU, 5-fluorouracil; CTA, citrazinic acid; DFP-11207, 5-chloro-2-(3-(3-(ethoxymethyl)-5-fluoro-2,6-dioxo-1,2,3,6-tetrahydopyrimidine-1-carbonyl) benzoyloxy)pyridine-4-yl-2,6-bis(propionyloxy)isonicotinate; PCA, percloric acid.
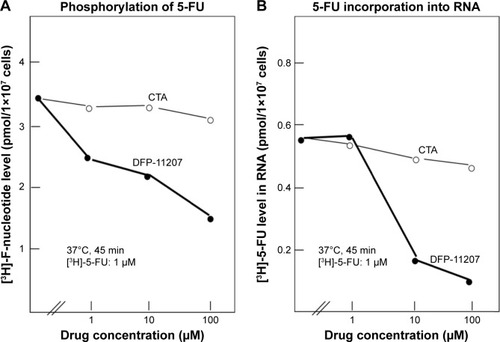
Figure 4 Conversion of EM-FU, a major metabolite of DFP-11207, to 5-FU by various liver microsomes.
Abbreviations: CTA, citrazinic acid; DFP-11207, 5-chloro-2-(3-(3-(ethoxymethyl)-5-fluoro-2,6-dioxo-1,2,3,6-tetrahydopyrimidine-1-carbonyl)benzoyloxy)pyridine-4-yl-2,6-bis(propionyloxy)isonicotinate; EM-FU, 1-ethoxymethyl-5-fluorouracil; 5-FU, 5-fluorouracil; FT, tegafur; HPLC, high-performance liquid chromatography.
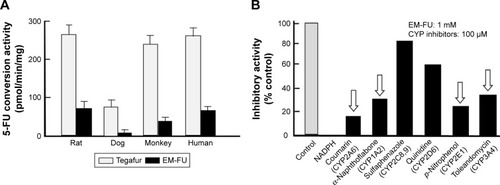
Figure 5 CTA and 5-FU levels in blood, small intestine, and tumor tissues of rats after oral administration of DFP-11207.
Abbreviations: CTA, citrazinic acid; DFP-11207, 5-chloro-2-(3-(3-(ethoxymethyl)-5-fluoro-2,6-dioxo-1,2,3,6-tetrahydopyrimidine-1-carbonyl)benzoyloxy)pyridine-4-yl-2,6-bis(propionyloxy)isonicotinate; 5-FU, 5-fluorouracil; HPLC, high-performance liquid chromatography.
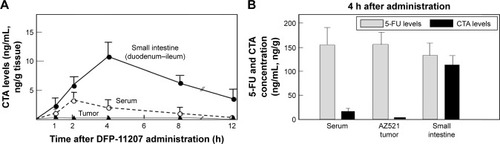
Figure 6 Comparative 5-FU levels in rat plasma following oral administration of DFP-11207 and S-1.
Abbreviations: DFP-11207, 5-chloro-2-(3-(3-(ethoxymethyl)-5-fluoro-2,6-dioxo-1,2,3,6-tetrahydopyrimidine-1-carbonyl)benzoyloxy)pyridine-4-yl-2,6-bis(propionyloxy)isonicotinate; 5-FU, 5-fluorouracil; HPLC, high-performance liquid chromatography; S-1, tegafur-gimeracil-oteracil.
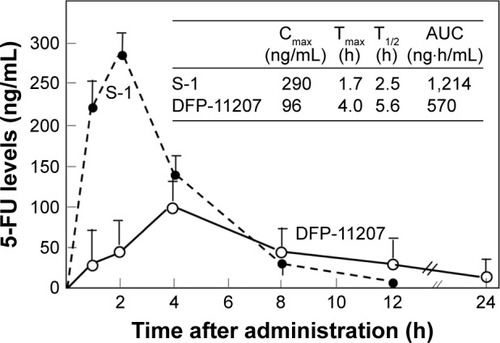
Figure 7 Antitumor activity and toxicity of DFP-11207 and S-1 in human colorectal cancer KM12C xenografts in nude rats.
Abbreviations: DFP-11207, 5-chloro-2-(3-(3-(ethoxymethyl)-5-fluoro-2,6-dioxo-1,2,3,6-tetrahydopyrimidine-1-carbonyl)benzoyloxy)pyridine-4-yl-2,6-bis(propionyloxy)isoni cotinate; S-1, tegafur-gimeracil-oteracil; WBC, white blood cell.
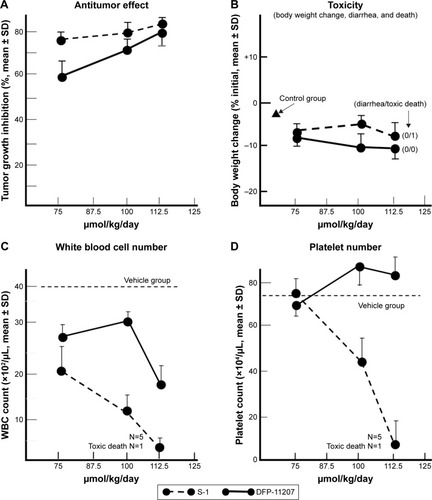
Figure 8 Histochemical evaluation of GI tissues (jejunum) in rats treated with each 112.5 µmol/kg of DFP-11207 and S-1.
Abbreviations: DFP-11207, 5-chloro-2-(3-(3-(ethoxymethyl)-5-fluoro-2,6-dioxo-1,2,3,6-tetrahydopyrimidine-1-carbonyl)benzoyloxy)pyridine-4-yl-2,6-bis(propionyloxy) isonicotinate; 5-FU, 5-fluorouracil; GI, gastrointestinal; S-1, tegafur-gimeracil-oteracil.
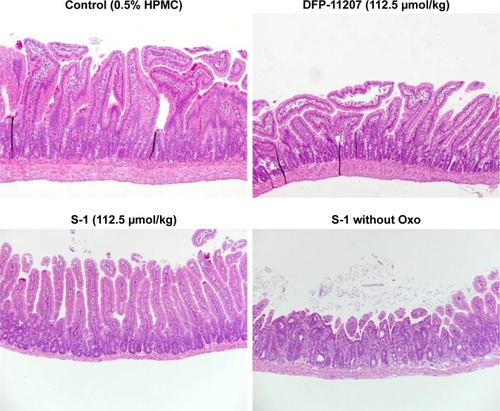
Table 2 Antitumor activity of DFP-1207 compared with 5-FU or gemcitabine on human gastrointestinal tumor xenografts in nude rats
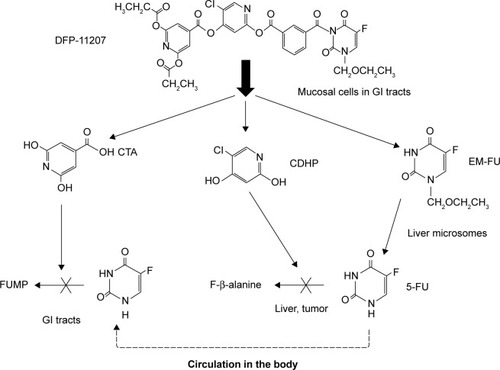
Figure 9 Possible biological metabolism and mechanism of action of DFP-11207 in rats.
Abbreviations: CDHP, 5-chloro-2,4-dihydroxypyridine; CTA, citrazinic acid; DFP-11207, 5-chloro-2-(3-(3-(ethoxymethyl)-5-fluoro-2,6-dioxo-1,2,3,6-tetrahydopyrimidine-1-carbonyl)benzoyloxy)pyridine-4-yl-2,6-bis(propionyloxy)isonicotinate; EM-FU, 1-ethoxymethyl-5-fluorouracil; 5-FU, 5-fluorouracil; GI, gastrointestinal; FUMP, 5-fluorouridine-5′-monophosphate.