Figures & data
Table 1 Demographic and baseline characteristics of the study population
Table 2 PK parameters of glimepiride after the administration of multiple oral doses of 4 mg glimepiride once per day (treatment G) and coadministration of 4 mg glimepiride and 20 mg rosuvastatin once per day (treatment GR) in healthy volunteers
Figure 1 Mean plasma concentration profiles of glimepiride at steady state.
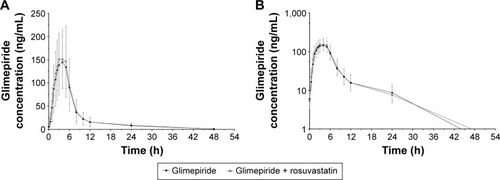
Table 3 PK parameters of rosuvastatin after administration of multiple oral doses of 20 mg rosuvastatin once per day (treatment R) and coadministration of 4 mg glimepiride and 20 mg rosuvastatin once per day (treatment GR) in healthy volunteers
Figure 2 Mean plasma concentration profiles of rosuvastatin at steady state.
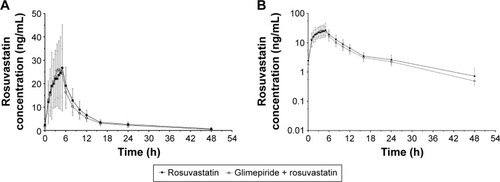
Table 4 PK parameters of glimepiride in the SLCO1B1 genotype and CYP2C9 diplotype group (part I)
Table 5 Pharmacokinetic parameters of rosuvastatin in the SLCO1B1 genotype group (part II)
Table 6 Incidence of adverse events per treatment group
