Figures & data
Table 1 Demographic characteristics of subjects (n=12)
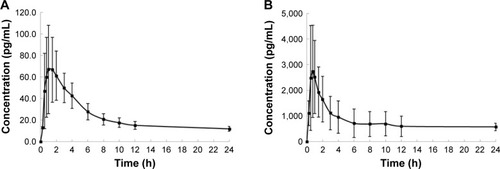
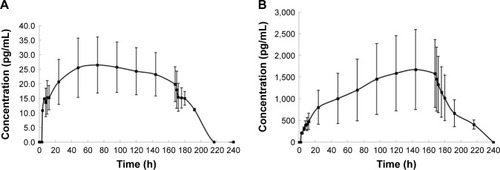
Table 2 The pharmacokinetic parameters of EE and GSD after a single-dose administration of 30 μg EE/75 μg GSD tablet and 7 days wearing of 1.2 mg EE/1.6 mg GSD study patch (n=12)
Table 3 The comparisons of main pharmacokinetic parameters of EE and GSD between a single-dose administration of 30 μg EE/75 μg GSD tablet and 7 days wearing of 1.2 mg EE/1.6 mg GSD study patch