Figures & data
Figure 1 Rationale used to design PDTM derivatives as tyrosinase inhibitors.
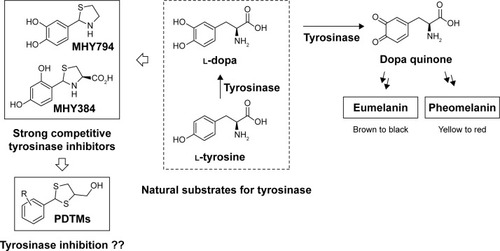
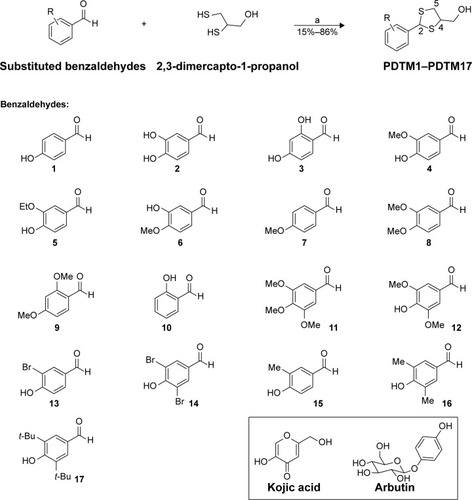
Table 1 Substitution patterns and tyrosinase-inhibitory activities by the synthesized compounds PDTM1–PDTM17, kojic acid, and arbutin
Table 2 Kinetic parameters of mushroom tyrosinase in the presence of PDTM3
Figure 2 Mode of mushroom tyrosinase inhibition by PDTM3.
Abbreviation: PDTM, (2-substituted phenyl-1,3-dithiolan-4-yl)methanol.
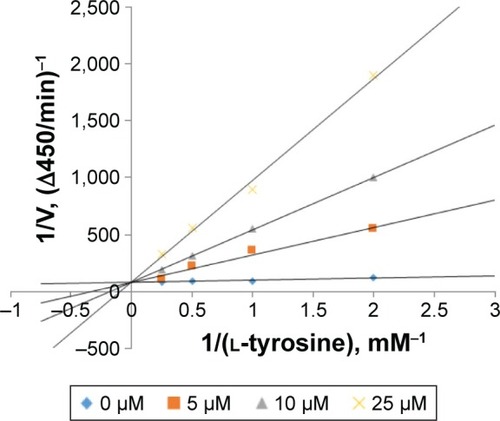
Figure 3 Docking simulation of PDTM derivatives and kojic acid with tyrosinase.
Abbreviation: PDTM, (2-substituted phenyl-1,3-dithiolan-4-yl)methanol.
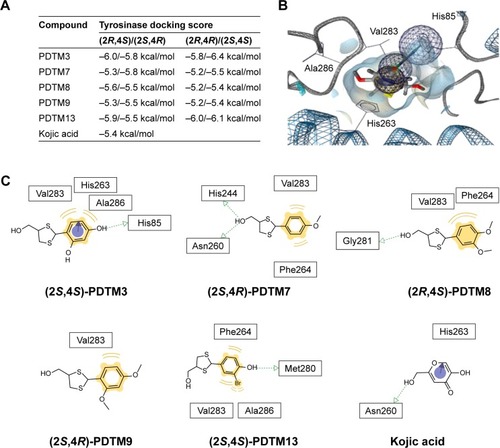
Figure 4 Effect of PDTM3 on B16F10 cell viability.
Abbreviation: PDTM, (2-substituted phenyl-1,3-dithiolan-4-yl)methanol.
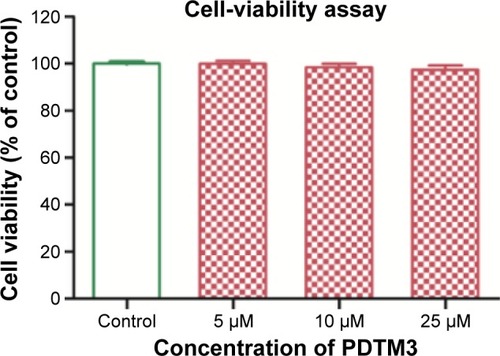
Figure 5 Antimelanogenic effect of PDTM3 in α-MSH-stimulated B16F10 cells.
Abbreviations: PDTM, (2-substituted phenyl-1,3-dithiolan-4-yl)methanol; MSH, melanocyte-stimulating hormone.
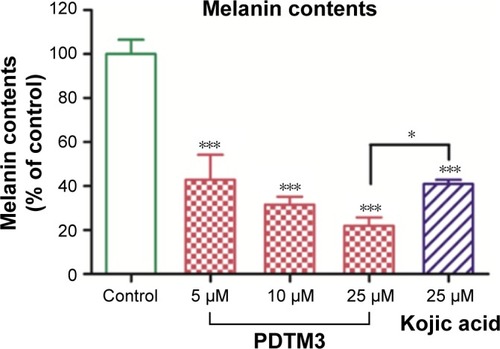
Figure 6 Effect of PDTM3 on tyrosinase activity in B16F10 cells.
Abbreviations: PDTM, (2-substituted phenyl-1,3-dithiolan-4-yl)methanol; MSH, melanocyte-stimulating hormone.
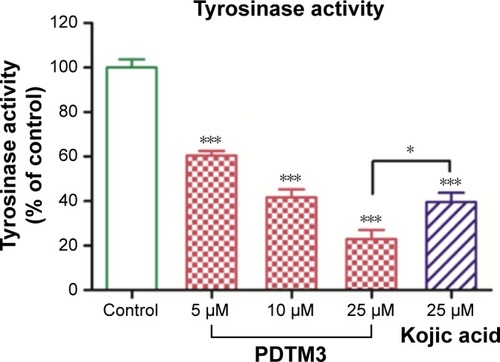
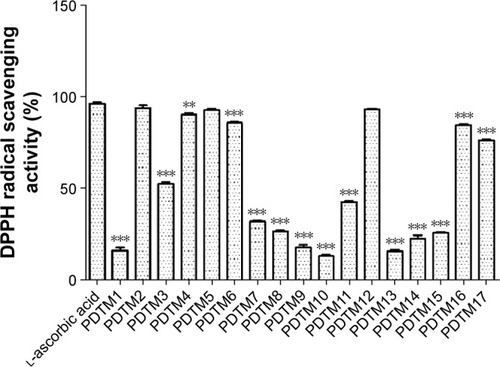
Figure 7 The effect of PDTM derivatives on DPPH radical scavenging activity.
Abbreviations: PDTM, (2-substituted phenyl-1,3-dithiolan-4-yl)methanol; DPPH, 2,2-diphenyl-1-picrylhydrazyl.