Figures & data
Table 1 Pharmacokinetic parameters of alendronic acid for each treatment/period in 36 healthy Chinese volunteers after a single oral dose of 70 mg of alendronate sodium
Figure 1 Plasma concentration–time curves of alendronic acid after a single oral dose of 70 mg of the test product (alendronate sodium) or the reference product (Fosamax®) (first and second doses) administered to 36 healthy Chinese male volunteers.
Abbreviation: SD, standard deviation.
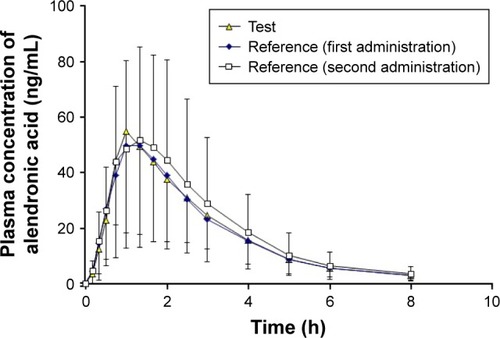
Table 2 Statistical analysis of the results obtained using the FDA’s RSABE method
Table 3 Statistical analysis of results obtained using the EMA’s RSABE method
Figure S1 Typical chromatograms of alendronic acid derivative (I) and d6-alendronic acid derivative (IS, II) in plasma.
Abbreviations: IS, internal standard; Rel int, relative intensity; MRM, multiple-reaction monitoring; LLOQ, low limit of quantification; XIC, extracted ion chromatogram.
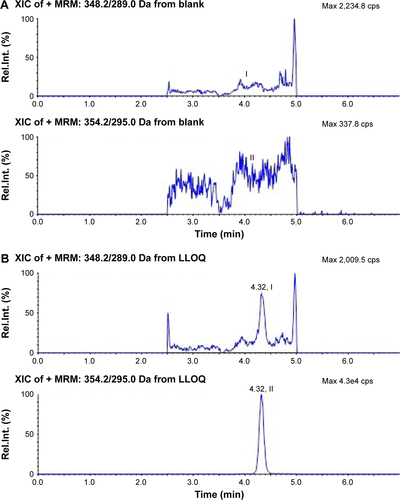
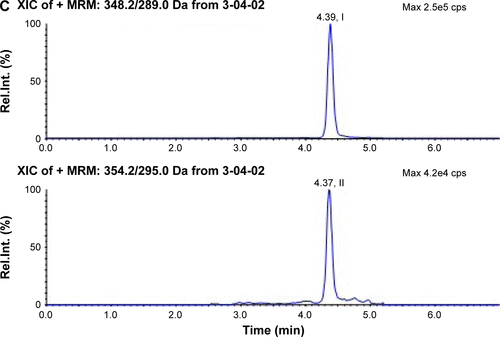
Table S1 Precision and accuracy of the LC–MS/MS method to determine alendronic acid in human plasma (in pre-study validation, n=3 days, six replicates per day)
