Figures & data
Figure 1 Scanning electron microscope image of the different microspheres.
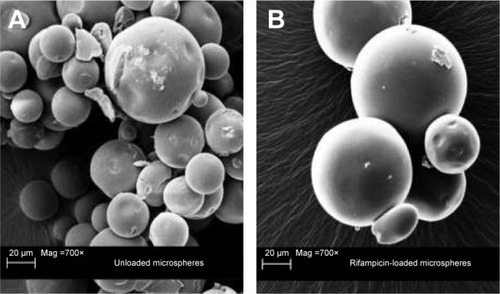
Figure 2 Measurement of the particle size distribution of the different manufactured microspheres.
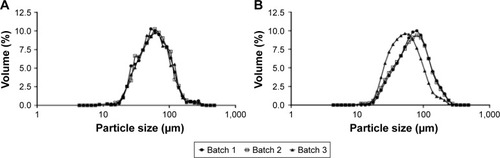
Figure 3 Investigation of the in vitro drug release from rifampicin-loaded microspheres.
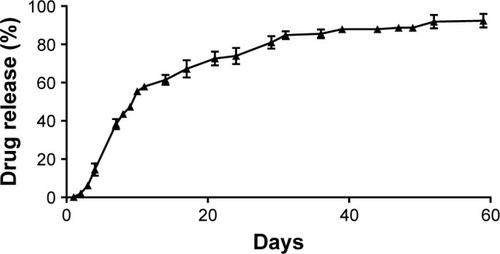
Figure 4 Agar diffusion test with unloaded and rifampicin-loaded microspheres.
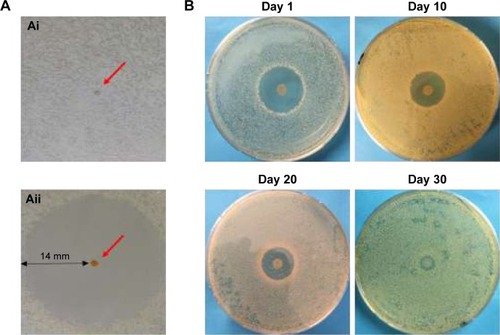
Figure 5 MTT assay of unloaded and rifampicin-loaded microspheres (n=3).
Abbreviation: SEM, scanning electron microscopy.
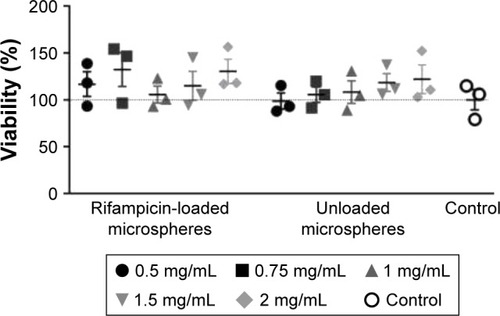
Figure 6 Scanning electron microscopy micrographs of different explanted mesh implants.
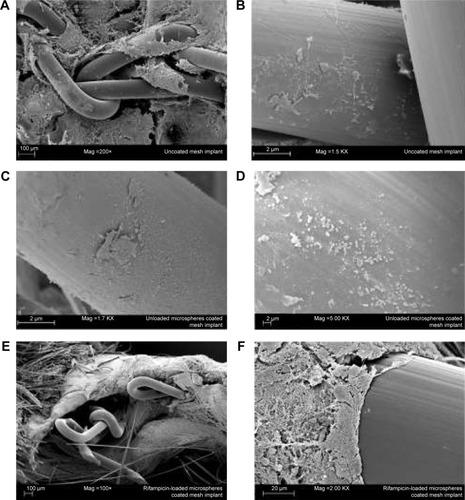
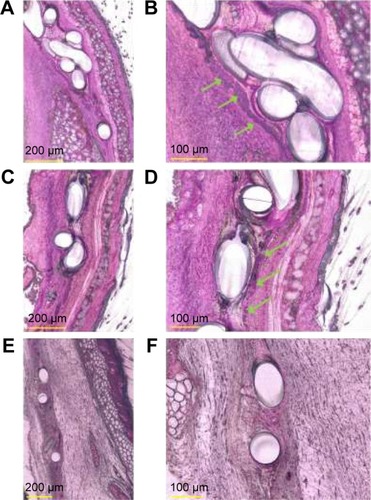
Figure 7 Histological evaluation of Technovit® 7200 imbedded, explanted mesh implants.