Figures & data
Figure 1 Comparative 30- and 54-week ASAS20 responses between infliximab innovator- and CT-P13-treated patients in the PLANETAS study.Citation26,Citation32
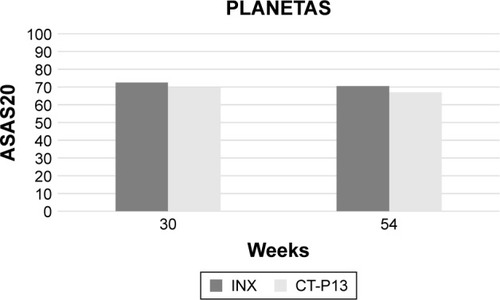
Figure 2 Comparative 30- and 54-week ACR20 response between infliximab innovator- and CT-P13-treated patients in the PLANETRA study.Citation27,Citation39
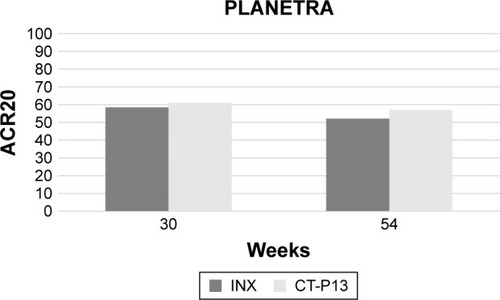
Table 1 PLANETRA and PLANETAS studies: open-label extension up to 102 weeks
Table 2 Real-life data on switching from infliximab RP to CT-P13
