Figures & data
Figure 1 Flowchart showing the selection of the treatment cohort.
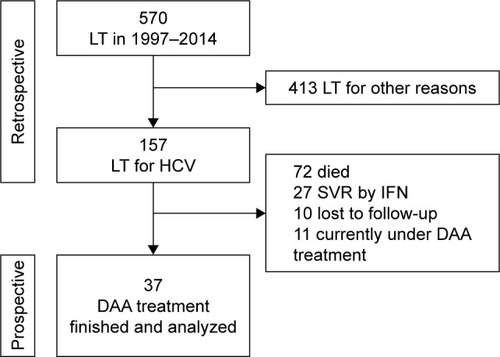
Figure 2 Survival of patients after liver transplantation with or without HCV (A) and GT1 vs non-GT1 (B).
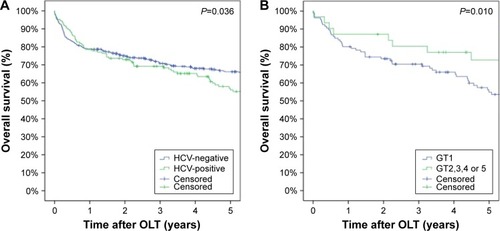
Table 1 Patient demographics at baseline (quantitative data are given as medians and interquartile range in parentheses)
Figure 3 Therapeutic regimens sorted by treatment duration.
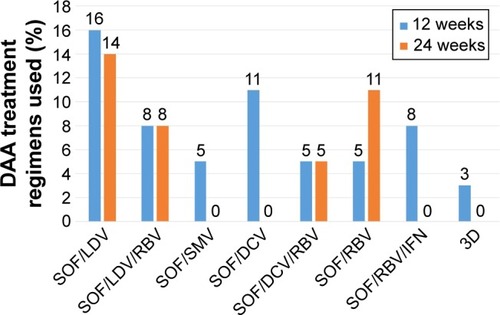
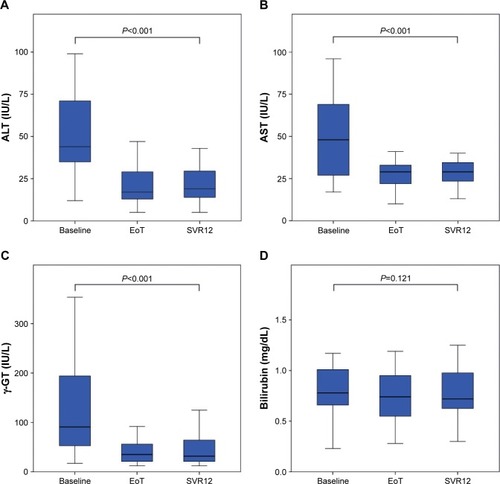
Figure 4 Biochemical response to DAA therapy. The activities of ALT (A), AST (B), γ-GT (C), and the serum levels of total bilirubin (D) declined within the observation period.