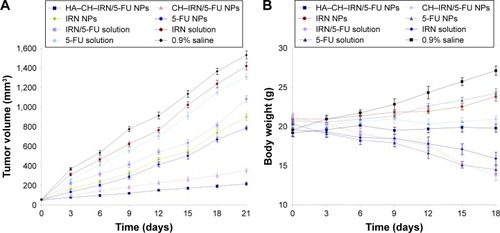
Figures & data
Figure 1 Scheme graph and TEM images of HA-CH-IRN/5-FU NPs and other NPs.
Abbreviations: HA, hyaluronic acid; CH, chitosan; IRN, irinotecan; 5-FU, 5-fluorouracil; NP, nanoparticle; PLGA, poly(d,l-lactide-co-glycolide); TEM, transmission electron microscopy.
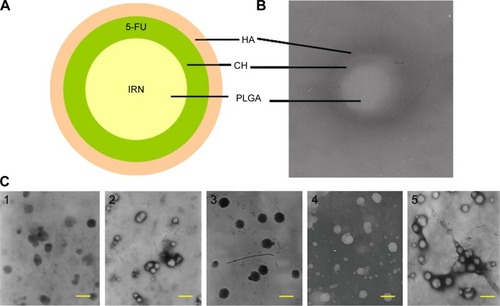
Table 1 Characterization of NPs
Figure 2 Changes in size in the presence of serum.
Abbreviations: HA, hyaluronic acid; CH, chitosan; IRN, irinotecan; 5-FU, 5-fluorouracil; NPs, nanoparticles.
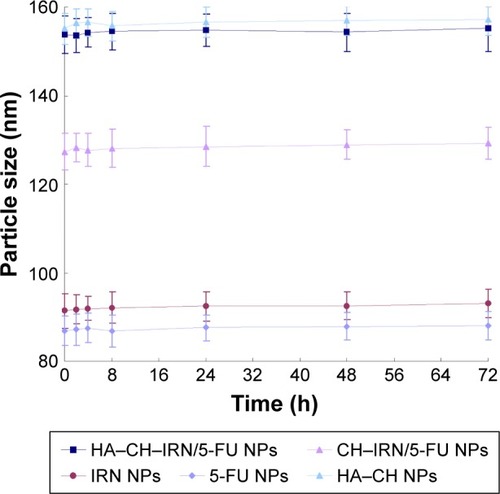
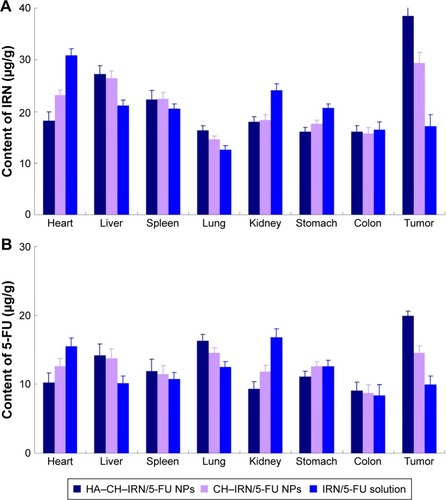
Figure 3 In vitro release of IRN (A) and 5-FU (B) from NPs.
Abbreviations: IRN, irinotecan; 5-FU, 5-fluorouracil; NPs, nanoparticles; HA, hyaluronic acid; CH, chitosan.
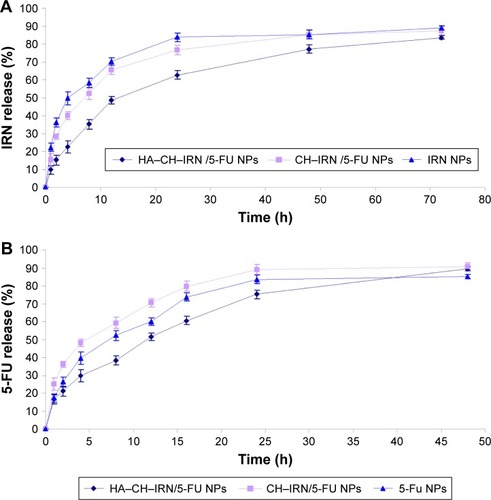
Figure 4 Cellular uptake efficiency of the NPs.
Abbreviations: NPs, nanoparticles; HA, hyaluronic acid; CH, chitosan; IRN, irinotecan; 5-FU, 5-fluorouracil.
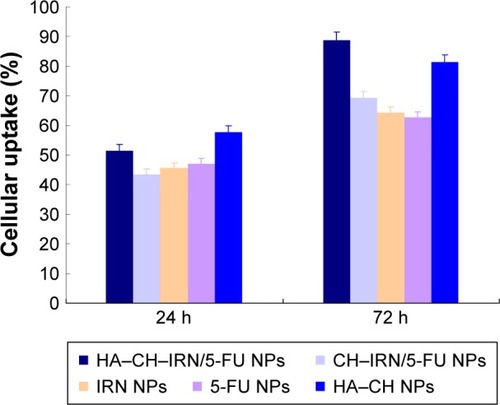
Figure 5 In vitro cytotoxicity of IRN and/or 5-FU-contained solutions or NPs investigated in MGC803 cells.
Abbreviations: IRN, irinotecan; 5-FU, 5-fluorouracil; NPs, nanoparticles; HA, hyaluronic acid; CH, chitosan.
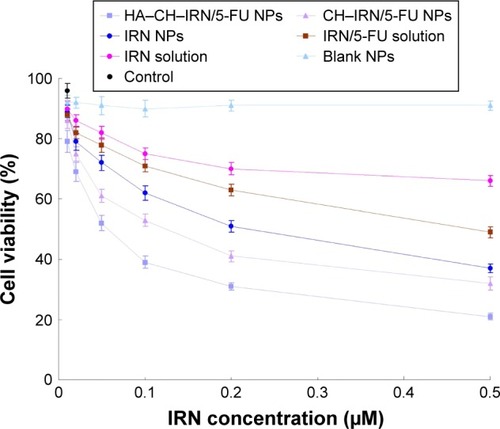
Table 2 Synergistic effect evaluation and selection of the ratio of drugs by CI calculation